KitchenChemist
Greenlighter
- Joined
- Apr 19, 2011
- Messages
- 18
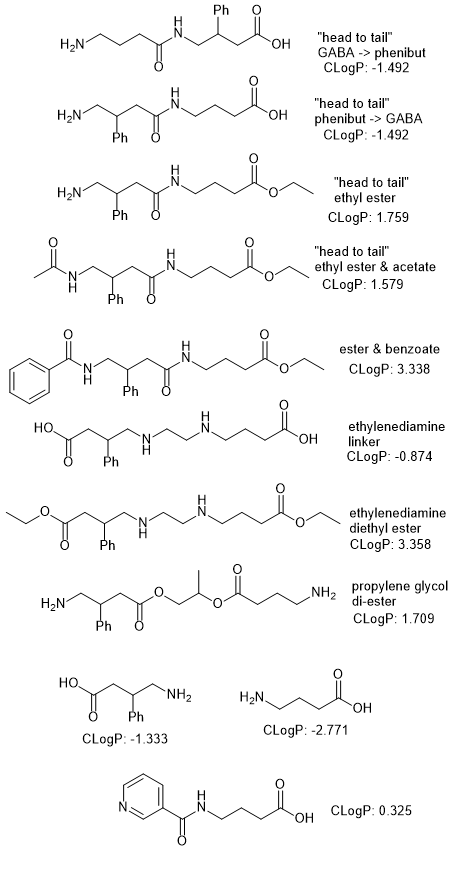
I am seeking the assistance of the members here with extensive chemistry knowledge. I have long used both Phenibut and Picamilon for Panic Attack Disorder and Generalized anxiety. Since Picamilon is essentially GABA bonded with Niacin, I got to thinking about what could be an anxiety super-drug.
The following chemical structures are Phenibut and GABA. We know that GABA supplements are useless, since GABA is produced in the brain, and orally taken GABA supplements do not cross the Blood-Brain-Barrier. However, I got to thinking.. I am looking for opinions on any way the following formulas could be combined in the same molecule (a molecule that would cross the BBB), that would separate back into Phenibut and GABA once in the brain.
Any insight would be greatly appreciated.


The following chemical structures are Phenibut and GABA. We know that GABA supplements are useless, since GABA is produced in the brain, and orally taken GABA supplements do not cross the Blood-Brain-Barrier. However, I got to thinking.. I am looking for opinions on any way the following formulas could be combined in the same molecule (a molecule that would cross the BBB), that would separate back into Phenibut and GABA once in the brain.
Any insight would be greatly appreciated.