Feretile
Bluelighter
- Joined
- Feb 2, 2022
- Messages
- 361
Fentanyl - Wikipedia
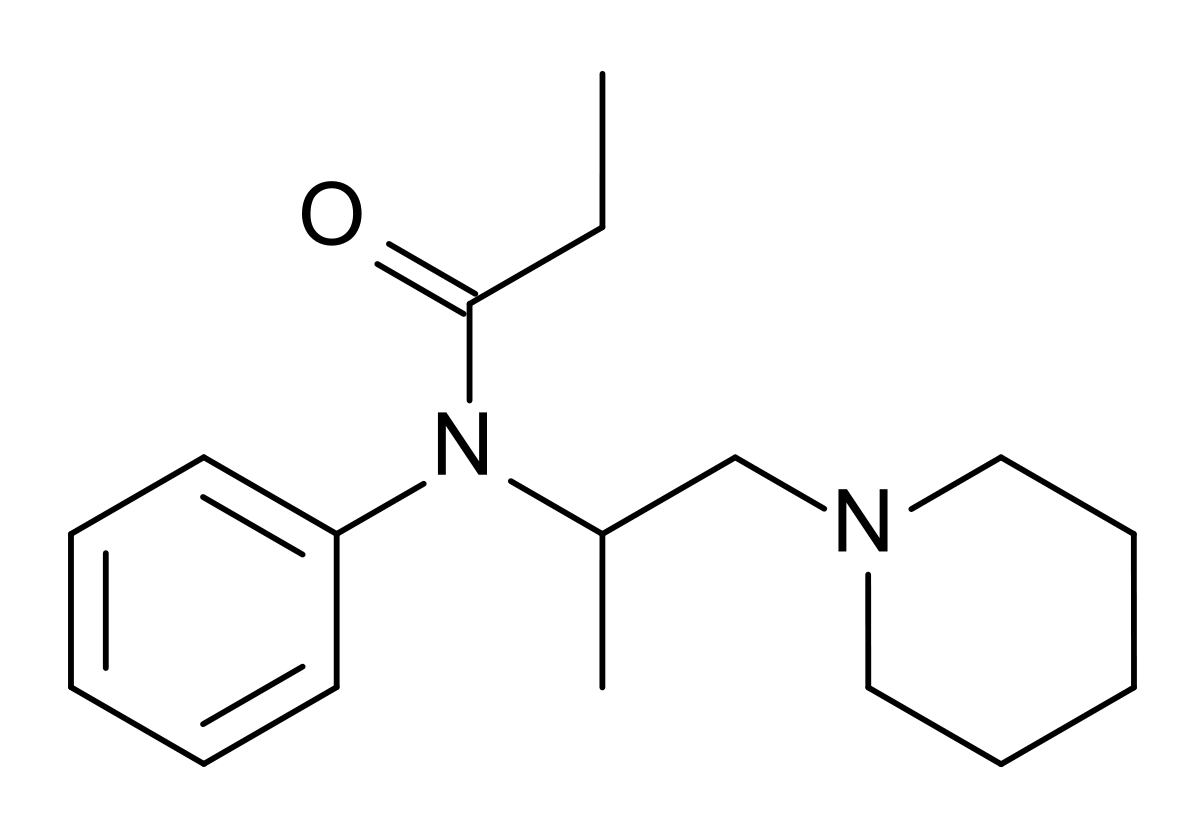
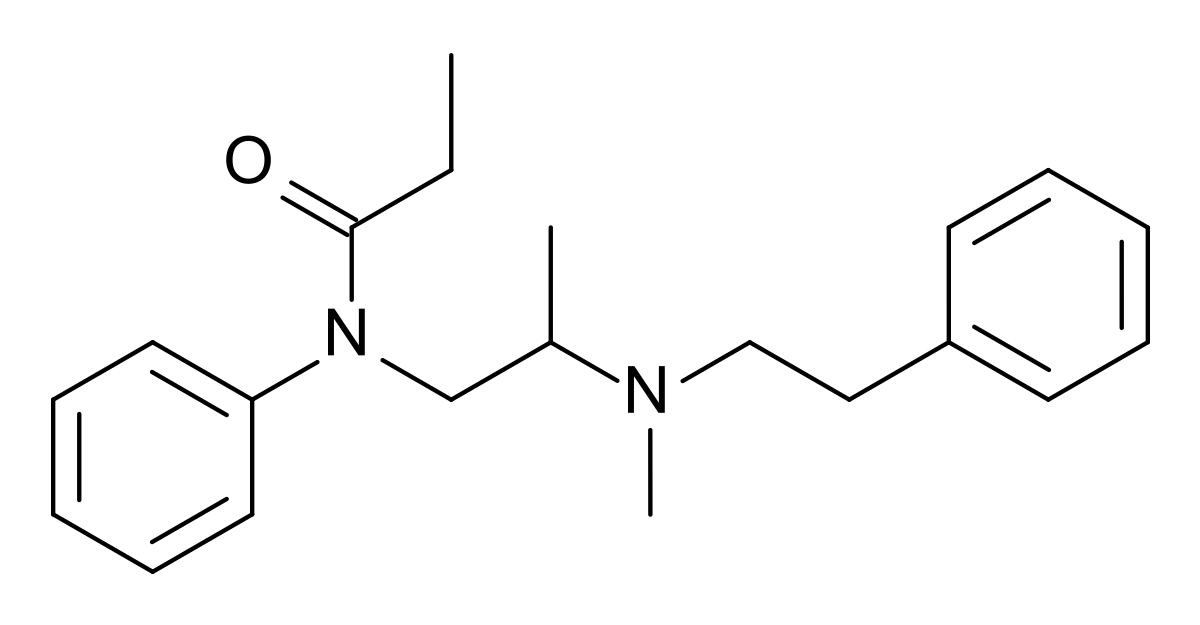
Phenampromide - Wikipedia
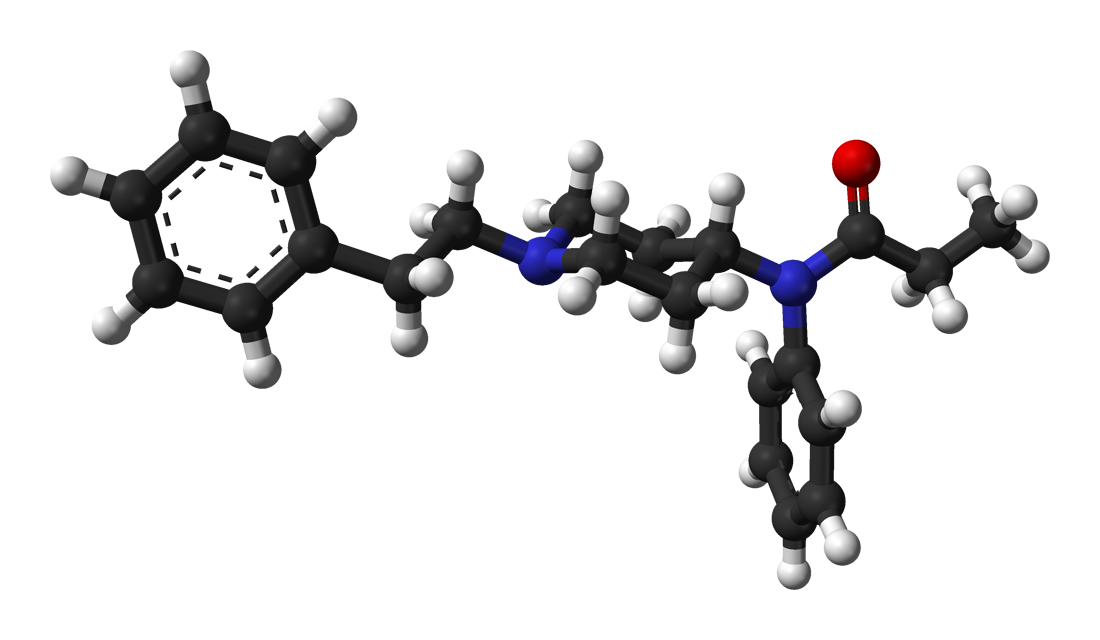
Diampromide - Wikipedia
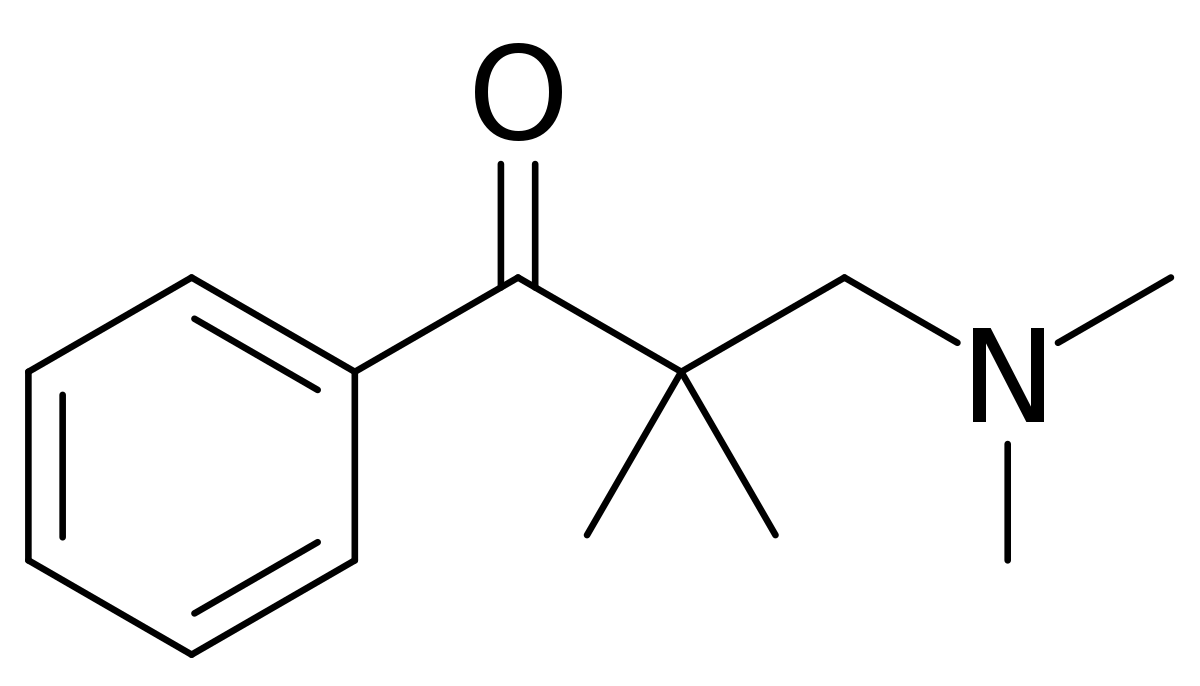
Dimethylaminopivalophenone - Wikipedia
If you take the trouble to overlay all 3 of the above compounds, you will note that in each case, the 2 benzene rings & the basic nitrogen overlay PRECICELY. Now, IMO fentanyl & 4-phenylphenapromide are equipotent although Janssen claims x80 while the American Cyamid Company only claims x60 for 4-phenylphenapromide. For diampromide they only claim it to be equipotent with morphine.
So is diampromide so much less potent? The answer is simply that it's compound with a flexible structure and in which the lowest energy state does not conform to the mu opioid receptors.. Still, in example 11 of the patent, they do give the p-Nitro homologue as an example. In other cases (p-nitro azaprocin, nitreridine & p-Nitro analogue of metofoline), the p-Nitro increases potency by an average of around x10 so ir's possible to produce sufficient potency for a synthetically simple opioid.
Since Dimethylaminopivalophenone &Methyl,((p-nitro)-2-phenylethylamino dimethylaminopivalophenone EACH take 1 step (although I suspect a custom precursor would have to be produced for the second), such simple synthesis (read cheap) for a LEGAL product that I guess is going to be about x10 M seems like a good target. So I canvass opinion. Any more opioids baring the (p-nitro)phenylethyl moiety would be appreciated. Of course a -OH on the beta carbon of the PE moiety increases potency around x2 (x4 if chiral).
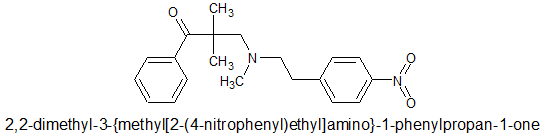
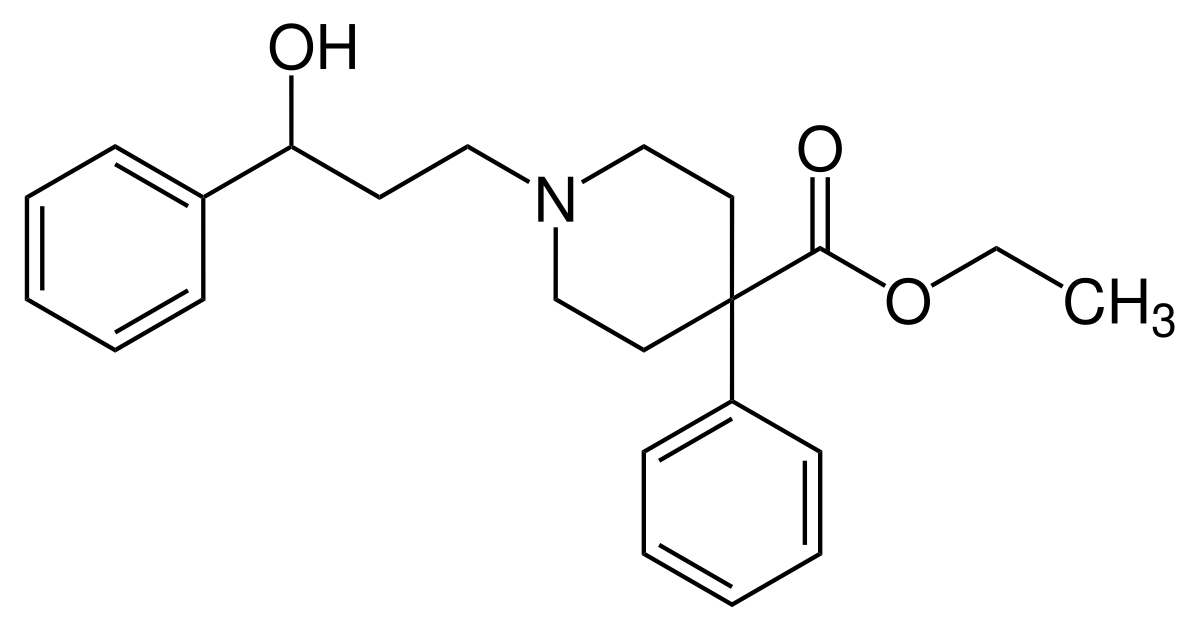
BTW actually, the p-Nitro is important because it increases the steric bulk. Without the -NO2 I would go for a 3-phenyl-3-hydroxypropyl moiety as seen in: