Any human reports online or is it that obscure?
So far couldnt find anything on human.. shitty thing is ppl use different development code for same molecule+common name. kind of confusing. FR did extensive animal testing (cf
here ).. getting very intrigued by that compound. From that FR patent, one the few stim as close as it gets to cocaine pharmacology (2x as potent! actually). might make it my next project, who knows.. will keep posted.
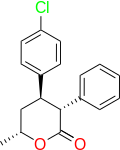
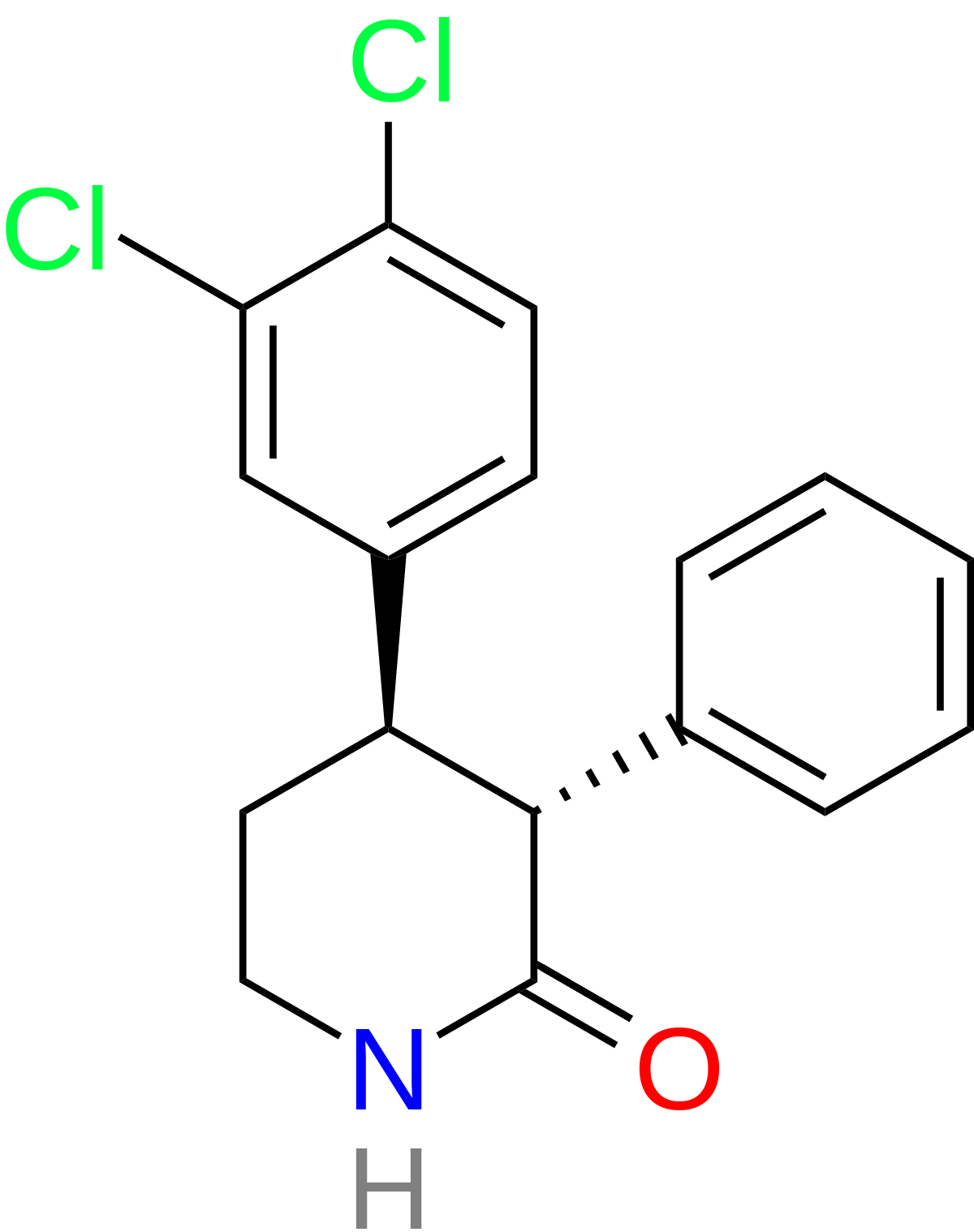
The OP mentions a compound with three chiral centres thus EIGHT enantiomers so it's only ever going to turn up if someone with the skill-set to take the bare-bones of the patent and go through the rather lengthy and complex synthesis. It was developed by a French company so their might be an obscure publication with further data,
But safe to say, their does exist a tiny fringe of grey-market labs (like BALCO) who specialize in such products. Of course, no independent QC is carried out and of course no safety trials so nobody knows if the stuff is safe...
Yes and No, only 2! or rather the synthetic route gives exclusively the 3,4 trans (3RS, 4RS) no cis. There is not difference in bioactivity between the 3R, 4R and 3S, 4S. The 5-methyl makes a difference tho: The psychostimulant activity reside pretty much all in the 5R methyl. so at most you have 2 psychoactives: trans(3R, 4R)-5R and trans(3S, 4S)-5S. Incidently, the active one (5R) is crystalline solid mp ca 80oC iirc which crystallize nicely out solvent while the inactive antipode is an oil that love to stay there which makes life so much more easier for ppl on those:
A Beautiful case of the proverbial Magic Methyl that makes medicinal chemists go ahhhhhh when Nature throw in garbage all SAR QSAR AI .. or whatever tool humans come up with to predict biology: Not only the compound without the methyl is not psychostimulant (It is a sedative!!!), the position of the methyl is critical (only one enantiomer 5R is psychostimulant), and one of the compound is an oil who likes to stick around in the solvent and the other (the psychoactive psychostimulant) is solid that crystallize out. Now this is on top of the fact the compound has no positive charge whatsover and retain potent activity unlike 99.9% psychostimulants known to Humans! check out the patent: US 4287206A
Oh BTW: that wiki page that says: "only the 3R, 4R, 5R is psychotherapeutic". actually both 3R, 4R, 5R and 3R, 4R, 5S are psychostimulants..the FR were trying dissociate that with antidepressant.. even that no much difference but you need something to take to market, right?