polymath
Bluelight Crew
- Joined
- Nov 4, 2010
- Messages
- 1,884
I was looking for alkylurea derivatives with sedative effects with Google (see Hammiltons bromisoval thread), when I found some articles about uridine derivatives that have hypnotic action...
The potent depressant effects of N3-phenacyluridine in mice.
Biol Pharm Bull. 1994 Apr;17(4):514-6.
In other papers about this subject it is claimed that compounds like phenacyluridine and benzyluridine are also active after intraperitoneal injection, but nothing is said about oral administration.
There's a hypothesis that the nucleoside derivatives exert their sedative action through some new unknown receptor, see http://www.journalsleep.org/Articles/240301.pdf .
So, apparently these compounds are hypnotics and also cause other signs of intoxication (motor incoordination). What makes this interesting is that things like benzyluridine would be easy to make from commercially available uridine by first protecting the hydroxyl groups by esterification and then reacting the ester with an appropriate electrophile. Of course these compounds are not necessarily 'recreational', a huge i.m. dose of haloperidol would probably cause sedation and motor incoordination too, without being fun in any way. No one has tested whether animals self-administer the nucleoside sedatives...
Is it likely that these compounds would be active orally, or would they be destroyed by first-pass metabolism?
Structure of phenacyluridine:
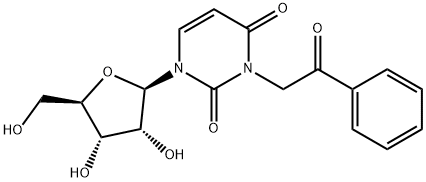
The potent depressant effects of N3-phenacyluridine in mice.
Biol Pharm Bull. 1994 Apr;17(4):514-6.
The hypnotic activity of N3-phenacyluridine in 2.0 mumol/mouse by intracerebroventricular (i.c.v.) injection was 20 times stronger than that of known N3-benzyluridine. In 0.5 mumol/mouse, i.c.v., this compound strongly potentiated both pentobarbital- and diazepam-induced sleep as compared to N3-substituted uridines, including N3-benzyluridine. Furthermore, the compound caused motor incoordination as well as decreasing spontaneous activity in the same dose. These results indicate that among the N3-substituted uridines and related compounds previously reported, N3-phenacyluridine possesses potent depressant effects.
In other papers about this subject it is claimed that compounds like phenacyluridine and benzyluridine are also active after intraperitoneal injection, but nothing is said about oral administration.
There's a hypothesis that the nucleoside derivatives exert their sedative action through some new unknown receptor, see http://www.journalsleep.org/Articles/240301.pdf .
So, apparently these compounds are hypnotics and also cause other signs of intoxication (motor incoordination). What makes this interesting is that things like benzyluridine would be easy to make from commercially available uridine by first protecting the hydroxyl groups by esterification and then reacting the ester with an appropriate electrophile. Of course these compounds are not necessarily 'recreational', a huge i.m. dose of haloperidol would probably cause sedation and motor incoordination too, without being fun in any way. No one has tested whether animals self-administer the nucleoside sedatives...
Is it likely that these compounds would be active orally, or would they be destroyed by first-pass metabolism?
Structure of phenacyluridine:

