Twisted_Chemist
Bluelighter
- Joined
- Mar 23, 2018
- Messages
- 21
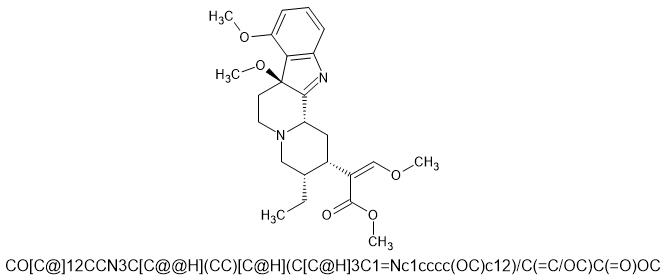
7-methylmitragynine, A new novel Mitragynine molecule. I am sorry if I put this in the wrong place. I could not find the advanced drug discussion page.
This is just a proposed molecule, something I have been playing around with in my spare time at work.
If a methyl group (-CH3) were added to the 7th position of mitragynine, the resulting compound would be a novel analog of mitragynine. 7-methylmitragynine, would have a structure similar to mitragynine but with a methyl group attached to the third ring at the 7th position.
This could potentially increase the molecule's lipophilicity. This, in turn, could affect the compound's absorption, distribution, metabolism, and excretion in the body.
NOTE:
This modification would likely alter the molecule's physical and chemical properties, as well as its pharmacological activity.
So far I am still running the CADD simulations, but it looking very promising.
And before people complain, I am not looking at making this to get high, but as a fun research project to kill time at work and see what I can come up with. Odds are, it will amount to nothing.
Any thoughts or suggestions on how to add the methyl group, please let me know! The hardest part (or most expensive if you buy it) is getting the pure mit.
The other option, would be to fully synth the chemical. Not cheap and no easy feat.
Thoughts?
----
ToDO's
1) Protect the functional groups on the molecule that should not be modified during the alkylation process.
2) Treat the protected mitragynine with a suitable alkylating agent, such as methyl iodide or dimethyl sulfate, under appropriate conditions to introduce the methyl group at the 7th position.
3)Remove the protecting groups to reveal the modified molecule.
This is just a proposed molecule, something I have been playing around with in my spare time at work.
If a methyl group (-CH3) were added to the 7th position of mitragynine, the resulting compound would be a novel analog of mitragynine. 7-methylmitragynine, would have a structure similar to mitragynine but with a methyl group attached to the third ring at the 7th position.
This could potentially increase the molecule's lipophilicity. This, in turn, could affect the compound's absorption, distribution, metabolism, and excretion in the body.
NOTE:
This modification would likely alter the molecule's physical and chemical properties, as well as its pharmacological activity.
So far I am still running the CADD simulations, but it looking very promising.
And before people complain, I am not looking at making this to get high, but as a fun research project to kill time at work and see what I can come up with. Odds are, it will amount to nothing.
Any thoughts or suggestions on how to add the methyl group, please let me know! The hardest part (or most expensive if you buy it) is getting the pure mit.
The other option, would be to fully synth the chemical. Not cheap and no easy feat.
Thoughts?
----
ToDO's
1) Protect the functional groups on the molecule that should not be modified during the alkylation process.
2) Treat the protected mitragynine with a suitable alkylating agent, such as methyl iodide or dimethyl sulfate, under appropriate conditions to introduce the methyl group at the 7th position.
3)Remove the protecting groups to reveal the modified molecule.
Last edited: