Feretile
Bluelighter
- Joined
- Feb 2, 2022
- Messages
- 361
Is anyone else familiar with the novel anoretic lorcaserin (Belviq)?
(1R)-8-CHLORO-1-METHYL-2,3,4,5-TETRAHYDRO-1H-3-BENZAZEPINE
US Patent 3716639A Anorexigenic tetrahydrobenzazepines
US Patent 3483185A N-substituted 2,3,4,5-tetrahydro-1h-3-benzazepines
it has hallucinogenic properties at higher than approved doses and users could develop psychiatric dependencies on the drug. (according to DEA). On 7 May 2013, DEA classified lorcaserin as a Schedule IV drug under the CSA act.
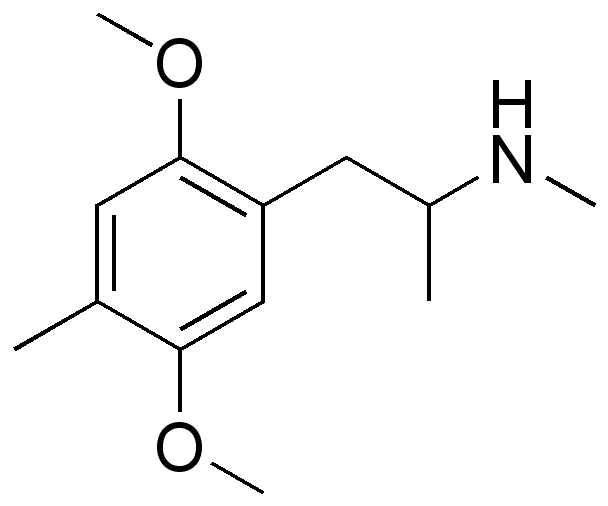
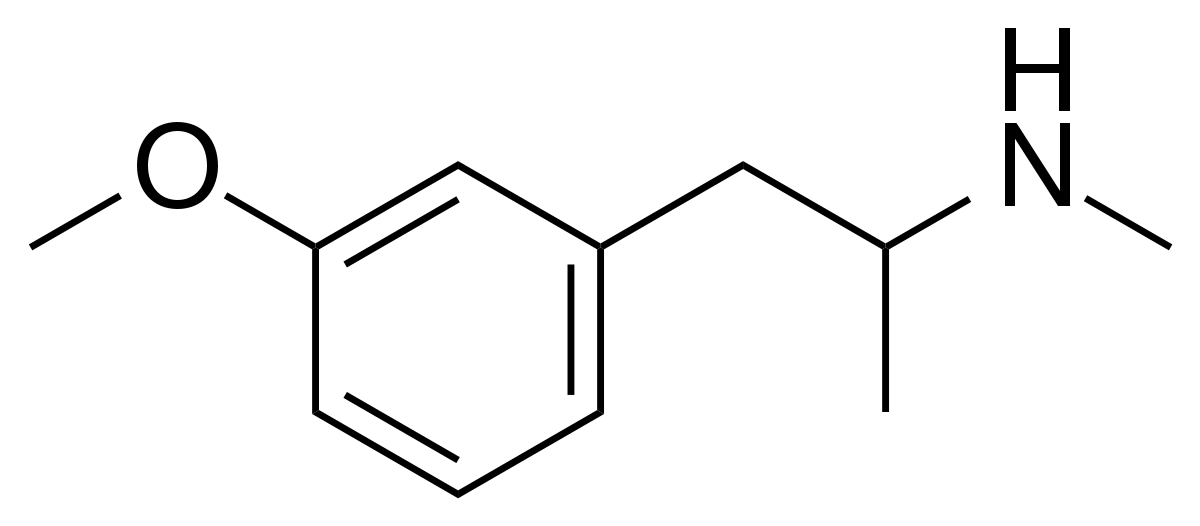
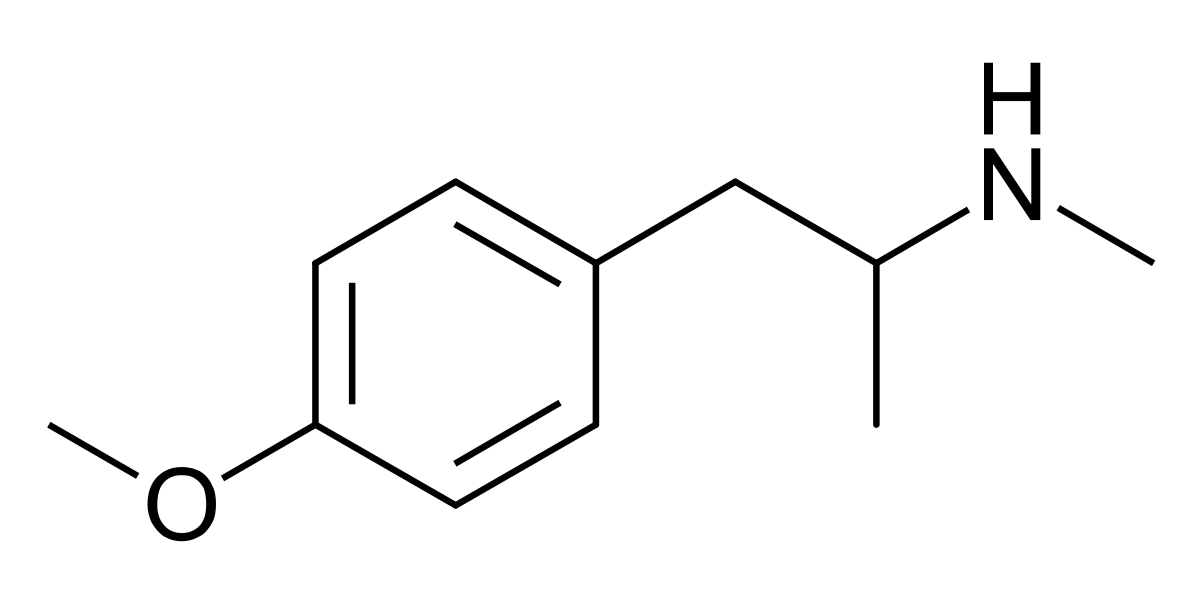
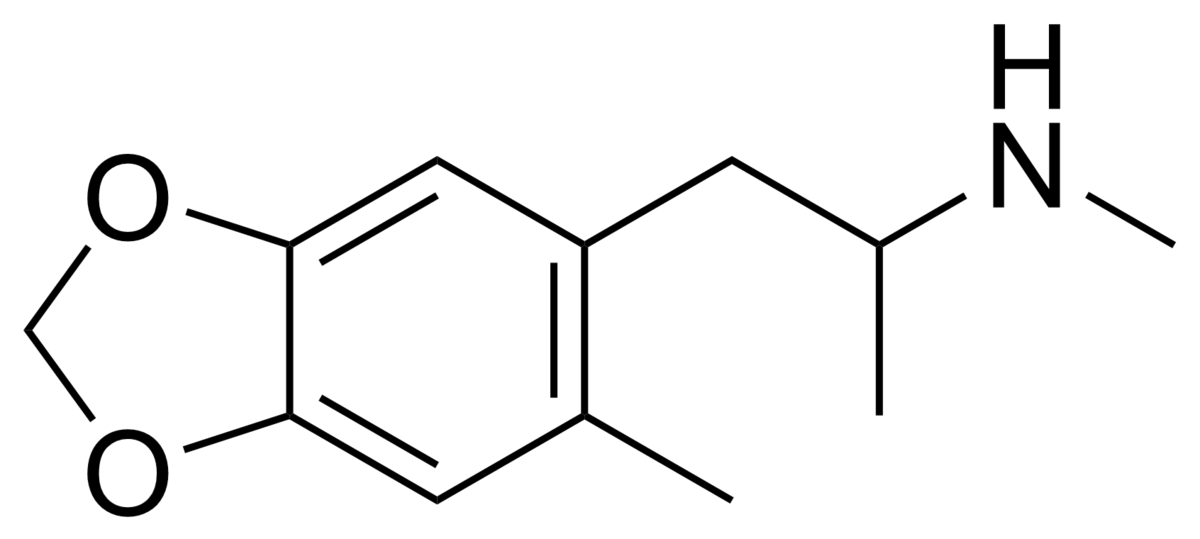
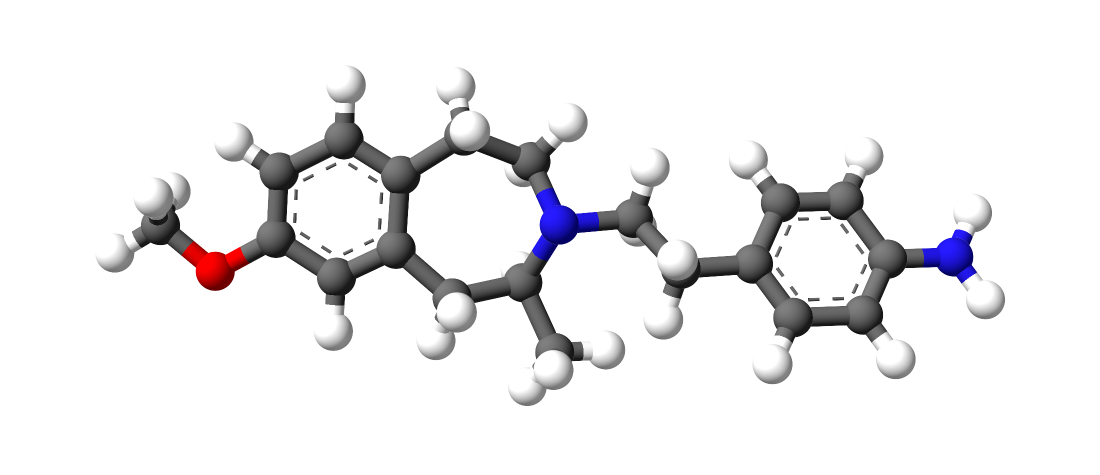
You will note that the benzapine ring, being 7-membered, has a 3D shape. If you overlay lorcvaserin & dexamphetamine, you will notice that the benzenes & amines ovcerlay PERFECTLY. I suspect that the methyl side-chain is to provide rigidity so that the benzapine ring will always 'bend' towards the (S).
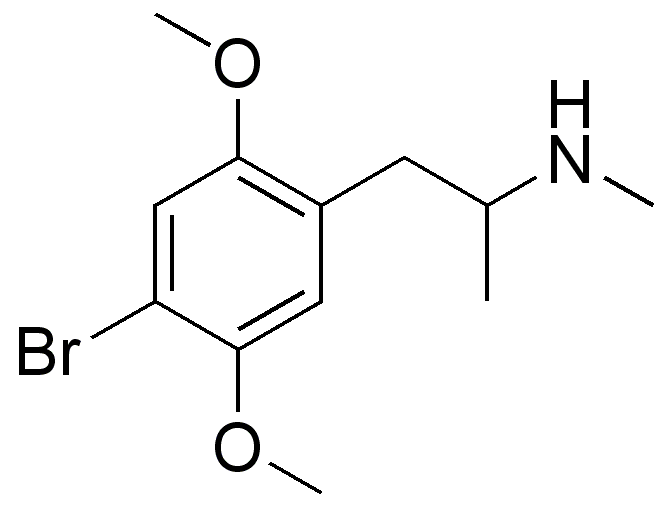
Even the early patents (and over 100 patents cover the compound stretching back almost 70 years. What they all have in common is that the position of the ring substitution is ALWAYS meta & is ALWAYS a halide.... in fact, in all post 1974 patents, it's always a chloro.
'Lorcaserin selectively activates the central serotonin 2C receptor over the 2A and 2B receptors and reduces appetite by binding to the 5HT-2C receptors on anorexigenic POMC neurons in the hypothalamus.'
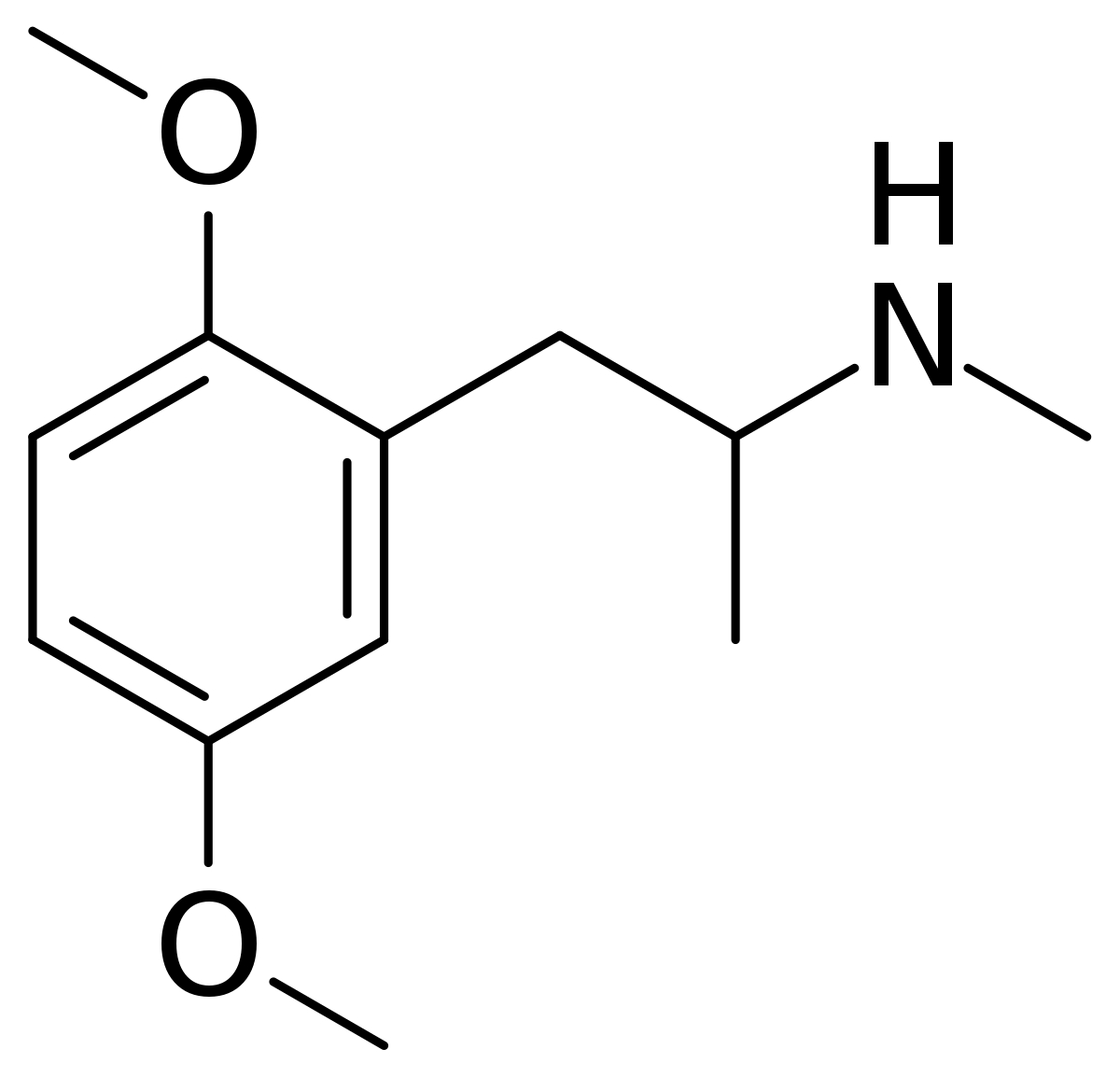
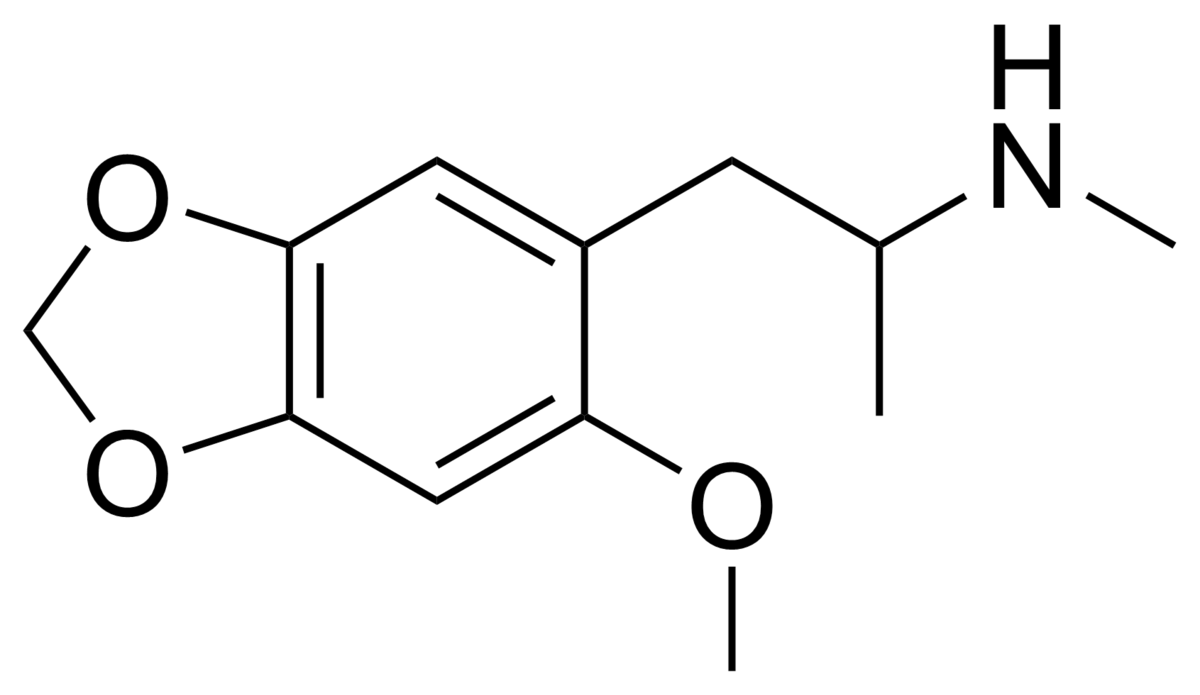
So, maybe tucked away in some paper is the QSAR of tetrahydrobenzapine 5HT2a affinity. Of course, recent research has seen -Cl ---> -OCH3 & the methyl on the benzapine ring being replacved by a phenyl ring. This lends NMDA affinity.....
Still, it's that age old dream. We discovered 5H2a binging tryptamines & PEAs in natural compounds. Ibogaine (and relatives) has a benzapine which, I am prepared to bet, overlays the N in aMT. I think the unpleasant effecvts of ibogaine are due to the 5-MeO moiety. I mean, 5-MeO DMT is just terrible & 5-MeO AMT can be deadly (and has been). As I have noted elsewhere, a 7-Methyl on a tryptamine ring always seems to improve subjective effects be they trippy or lovey.
So - anybody else prepared to put in the hours looking for more benzapines with 5HT2a affinity. The above represents many hours of work.
(1R)-8-CHLORO-1-METHYL-2,3,4,5-TETRAHYDRO-1H-3-BENZAZEPINE
US Patent 3716639A Anorexigenic tetrahydrobenzazepines
US Patent 3483185A N-substituted 2,3,4,5-tetrahydro-1h-3-benzazepines
it has hallucinogenic properties at higher than approved doses and users could develop psychiatric dependencies on the drug. (according to DEA). On 7 May 2013, DEA classified lorcaserin as a Schedule IV drug under the CSA act.
You will note that the benzapine ring, being 7-membered, has a 3D shape. If you overlay lorcvaserin & dexamphetamine, you will notice that the benzenes & amines ovcerlay PERFECTLY. I suspect that the methyl side-chain is to provide rigidity so that the benzapine ring will always 'bend' towards the (S).
Even the early patents (and over 100 patents cover the compound stretching back almost 70 years. What they all have in common is that the position of the ring substitution is ALWAYS meta & is ALWAYS a halide.... in fact, in all post 1974 patents, it's always a chloro.
'Lorcaserin selectively activates the central serotonin 2C receptor over the 2A and 2B receptors and reduces appetite by binding to the 5HT-2C receptors on anorexigenic POMC neurons in the hypothalamus.'
So, maybe tucked away in some paper is the QSAR of tetrahydrobenzapine 5HT2a affinity. Of course, recent research has seen -Cl ---> -OCH3 & the methyl on the benzapine ring being replacved by a phenyl ring. This lends NMDA affinity.....
Still, it's that age old dream. We discovered 5H2a binging tryptamines & PEAs in natural compounds. Ibogaine (and relatives) has a benzapine which, I am prepared to bet, overlays the N in aMT. I think the unpleasant effecvts of ibogaine are due to the 5-MeO moiety. I mean, 5-MeO DMT is just terrible & 5-MeO AMT can be deadly (and has been). As I have noted elsewhere, a 7-Methyl on a tryptamine ring always seems to improve subjective effects be they trippy or lovey.
So - anybody else prepared to put in the hours looking for more benzapines with 5HT2a affinity. The above represents many hours of work.
Last edited: