Nagelfar
Bluelight Crew
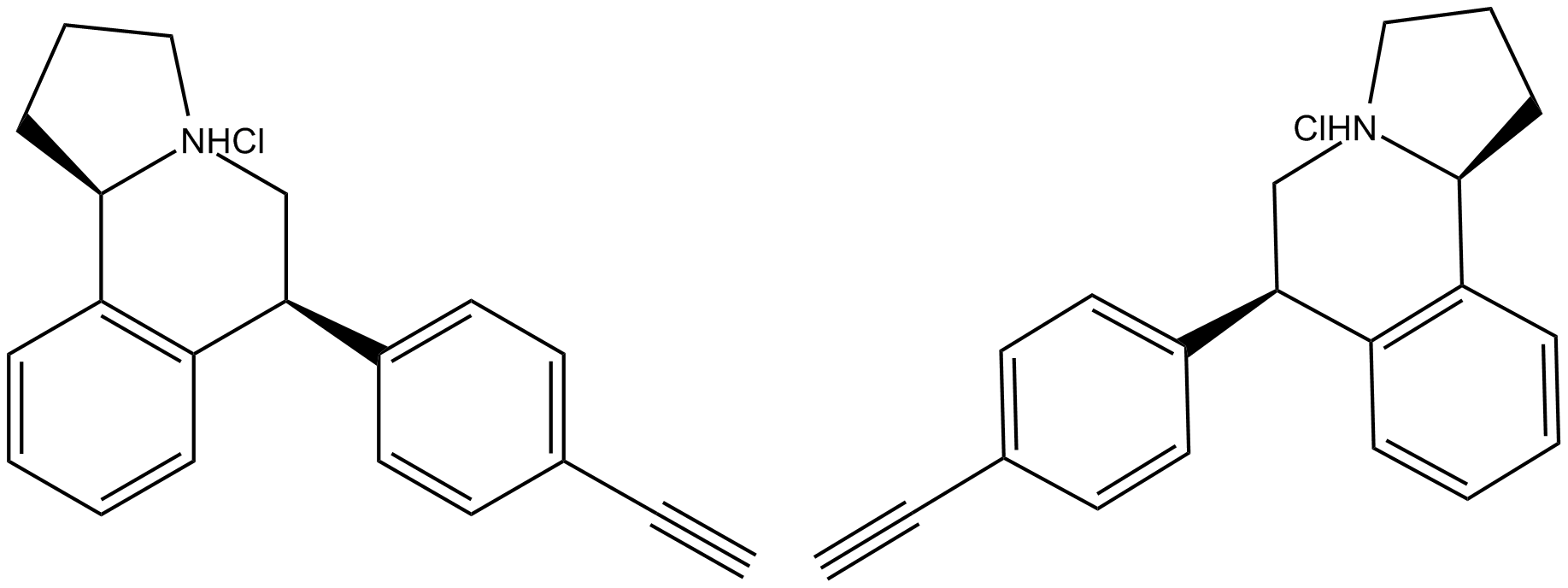
^Notice the NHCl (from the opposite facing flipping to ClNH)
JNJ-7925476, the image is so small I didn't notice it at first. Is an NHCl group possible? I've never seen it and the molecular rendering program I tried it on (that allows you to drop atoms on top of one another to combined them) doesn't allow this (it replaces the atom if it doesn't naturally adhere)



