(zonk)
Bluelighter
- Joined
- May 24, 2008
- Messages
- 687
When looking into methamphetamine cuts and how to identify their effects an idea came to me and when I searched it a site that specializes in providing cuts actually stocked this, (but in another , not with cuts section ). My question of course is obviously what effects would it have to offer in regards to SAR speculation?
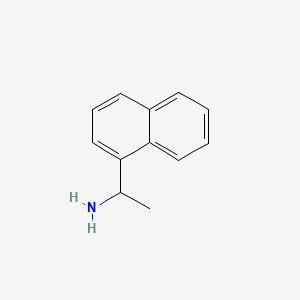
 pubchem.ncbi.nlm.nih.gov
Structure
pubchem.ncbi.nlm.nih.gov
Structure
1-(1-Naphthyl)ethylamine
It's sort of a hybrid of amphetamine and isopropylbenzylamine and I guess naphthylaminopropane if you want to take it there...
1-(1-Naphthyl)ethylamine
1-(1-Naphthyl)ethylamine | C12H13N | CID 98089 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.
1-(1-Naphthyl)ethylamine
It's sort of a hybrid of amphetamine and isopropylbenzylamine and I guess naphthylaminopropane if you want to take it there...
