paracelsius
Bluelighter
- Joined
- Mar 11, 2020
- Messages
- 197
this from pubchem db:
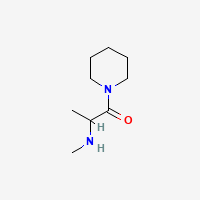
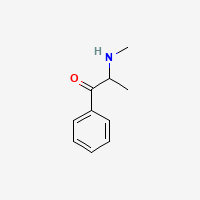
sure it looks like the equivalent of propylhexedrine/meth pair
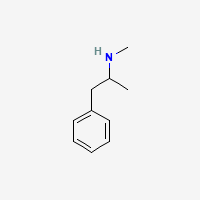
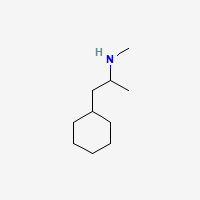
for cathinones

with the aryl ring replaced by a piperidine..(sorry that stupid program likes to draw it upside down when switching from phenyl to piperidyl. .but you got the point!
.but you got the point!
so is it analog laws jurisdictions they talking about?? or is it specifically scheduled?
any link for infos would be appreciated... thx
anybody knows which jurisdictions .. cant find any info anywhere on legal status of that one..This is a regulated substance. Complete access to the full Controlled Substances Squared application for 24 hours - Scitegrity database ....compound controlled in 7 jurisdictions
sure it looks like the equivalent of propylhexedrine/meth pair
for cathinones
with the aryl ring replaced by a piperidine..(sorry that stupid program likes to draw it upside down when switching from phenyl to piperidyl.
so is it analog laws jurisdictions they talking about?? or is it specifically scheduled?
any link for infos would be appreciated... thx