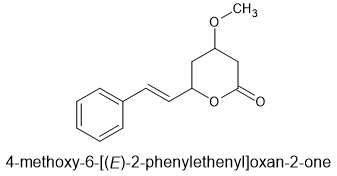
If you look, I've just read all the papers and patents in which the opioid activity of tianeptine is screened. One example proved to have a MOR affinity >160 times that of tianeptine.
It's useful for those seeking to build a training set but the thing to beware of is the unknown. we don't know if 7b is, like tianeptine, a superagonist. It might be an agonist, partial agonist, silent agonist, inverse agonist or antagonist. But a superagonist with a MOR Ki of just 1.2 represents a potentially dangerous compound.