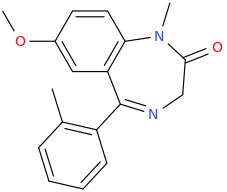
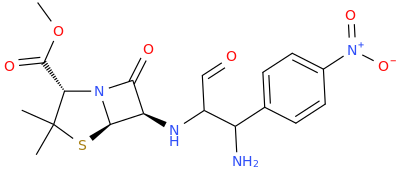
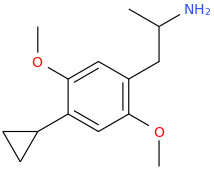
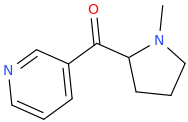
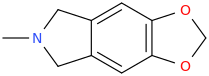
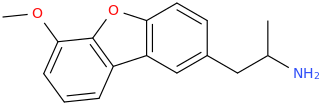
-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Ketamine salts solubility
- Thread starter fastandbulbous
- Start date
- Status
- Not open for further replies.
AlsoTapered
Bluelighter
- Joined
- Apr 1, 2023
- Messages
- 3,076
I am informed that somebody has found a way to use the above that does not proceed by concerting it into PMK first. I've spent a year looking. Anyone found it yet?
Gaffy
Bluelighter
- Joined
- Oct 27, 2018
- Messages
- 1,210
Neuropeptide FF 1 & 2 , off my own works. Worth trillions in advance.
 swisstargetprediction.ch
swisstargetprediction.ch
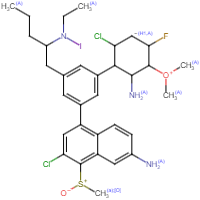
@sekio @SantaClaus
The best GaffyRC's has to offer.
SwissTargetPrediction

@sekio @SantaClaus
The best GaffyRC's has to offer.
AlsoTapered
Bluelighter
- Joined
- Apr 1, 2023
- Messages
- 3,076
Won't those formal charges ensure it's going to be unstable? Like instantly?
AlsoTapered
Bluelighter
- Joined
- Apr 1, 2023
- Messages
- 3,076
OxyNeoetorphine codone …oxy and etorphine mash up
Won't be very potent - the 6 methoxy interacts with the -OH. MAYBE that O-methyl moves between the two spots?
N-CF3?
Won't ionize.
LucidSDreamr
Bluelighter
- Joined
- May 23, 2013
- Messages
- 7,349
don’t need to ionize opioids to use them. See No. 3 heroinWon't ionize.
AlsoTapered
Bluelighter
- Joined
- Apr 1, 2023
- Messages
- 3,076
Won't be very potent - the 6 methoxy interacts with the -OH on that side-chain. MAYBE that O-methyl moves between the two spots?
N-CF3?
Won't ionize.
Why has nobody suggested an N-phenylethyl or homologue. I've posted a link to the hundreds of levophhanol analouges made and tested. The N-phenylethyl was x80M, the N-2-(2-thienyl)ethyl x240M and N-2-(2-furanyl)ethyl x480M.
don’t need to ionize opioids to use them. See No. 3 heroin
The RECEPTOR requires a positively ionizable function - I've posted multiple papers on this.
LucidSDreamr
Bluelighter
- Joined
- May 23, 2013
- Messages
- 7,349
Sorry I don’t read every paper you post on BL. Please forgive me.Why has nobody suggested an N-phenylethyl or homologue. I've posted a link to the hundreds of levophhanol analouges made and tested. The N-phenylethyl was x80M, the N-2-(2-thienyl)ethyl x240M and N-2-(2-furanyl)ethyl x480M.
The RECEPTOR requires a positively ionizable function - I've posted multiple papers on this.
I was stoned off my ass and made up chemdraw structures without reading shit about SAR.
Last edited:
AlsoTapered
Bluelighter
- Joined
- Apr 1, 2023
- Messages
- 3,076
But lets go through it as I did, beginning with the patent.
US 3904641 - Triazolothienodiazepine compounds by Michio Nakanishi, Tetsuya, Tahara Kazuhiko Araki & Masami Shiroki
When you have read, understood and digested that, we shall proceed. Because I'm 100% going to ensure you do the work I did.
US 3904641 - Triazolothienodiazepine compounds by Michio Nakanishi, Tetsuya, Tahara Kazuhiko Araki & Masami Shiroki
When you have read, understood and digested that, we shall proceed. Because I'm 100% going to ensure you do the work I did.
AlsoTapered
Bluelighter
- Joined
- Apr 1, 2023
- Messages
- 3,076
I don't use drugs other than those prescribed by my doctor. I'm not addicted to them although they do both produce a defined abstinence syndrome. I don't drink alcohol although I do smoke tobacco occasionally.
BUT having spent 8 years of higher education in the subject and now being severely disables does mean I have a huge amount of time to expend.
BUT having spent 8 years of higher education in the subject and now being severely disables does mean I have a huge amount of time to expend.
If anybody wants near pure LAH, all they have to do is obtain a fungus called Metarhizium brunneum and culture it on agar. LAH probably metabolizes into LAA[1] (although someone posted an interesting argument against this[2]), however, perhaps Peter Webster's LAA equlibrium hypothesis should be taken into account before dismissing LAH.[3]
The fungus Metarhizium brunneum produces ergot alkaloids of the lysergic acid amide class, most abundantly lysergic acid α-hydroxyethylamide (LAH).
...the plant root symbiont and insect pathogen Metarhizium brunneum produces ergonovine and LAH, with the concentration of LAH dwarfing that of ergonovine by approximately 200 to 1[5, 6].
Contribution of a novel gene to lysergic acid amide synthesis in Metarhizium brunneum. Britton, K.N., Steen, C.R., Davis, K.A., Sampson, J.K., Panaccione, D.G. BMC Res Notes 15, 183 (2022). doi: 10.1186/s13104-022-06068-2
Isolates of M. brunneum and M. anisopliae accumulated the lysergic acid amides lysergic acid α-hydroxyethyl amide, ergine, and ergonovine on sucrose-yeast extract agar but not on two other tested media.
Metarhizium spp. produce ergot alkaloids in culture in an isolate-specific manner. Three of 10 tested isolates of M. brunneum accumulated the ergot alkaloids lysergic acid α-hydroxyethylamide (LAH) and its degradation product ergine, as well as lesser quantities of ergonovine, when grown on sucrose-yeast extract agar (SYE) (Fig. 3A; Table 1). These alkaloids were identified by their fluorescence properties and retention times relative to an authentic standard (for ergonovine) or standards prepared from Periglandula sp.-infected Ipomoea leptophylla (LAH and ergine) (39).
The accumulation of ergot alkaloids in M. brunneum depended on the culture medium on which the fungus was grown; isolates of M. brunneum produced ergot alkaloids on SYE but not on corn meal agar or malt extract agar.
All ergot alkaloid-positive species of Metarhizium produced lysergic acid amides: most abundantly LAH (along with its hydrolysis product ergine) and lesser quantities of ergonovine. LAH occurs in four stereoisomers as documented in previous work with Claviceps pasapali (42).
Interestingly, M. brunneum ARSEF 9354 secreted the vast majority of its ergot alkaloids into the culture medium. In this way it differs from other ergot alkaloid-producing fungi, such as N. fumigata and Claviceps purpurea, which retain much of their ergot alkaloids in their mycelia, complicating extraction and purification.
Note: This is a wild strain of M. brunneum, not a GMO.
Several additional Metarhizium species, including M. acridum (two isolates), M. album (three isolates), M. anisopliae (four isolates), M. flavoviride (one isolate), M. guizhouense (two isolates), M. majus (two isolates), M. pingshaense (one isolate), and M. robertsii (two isolates), were tested for accumulation of ergot alkaloids on SYE. Among these additional isolates, only M. anisopliae ARSEF 7426 accumulated detectable ergot alkaloids on SYE when analyzed by HPLC with fluorescence detection (Table 1).
Several Metarhizium Species Produce Ergot Alkaloids in a Condition-Specific Manner. Leadmon CE, Sampson JK, Maust MD, Macias AM, Rehner SA, Kasson MT, Panaccione DG. Appl Environ Microbiol. 2020 Jul 2;86(14):e00373-20. doi: 10.1128/AEM.00373-20
Further Reading: Metarhizium: jack of all trades, master of many. St. Leger RJ, Wang JB. Open Biology, Dec 9, 2020. doi: 10.1098/rsob.200307
1. LAH stability info
2. https://www.reddit.com/r/LSA/commen.../?utm_source=share&utm_medium=web2x&context=3 (see last quote)
3. So, we have the possibility that a molecule of ergine [LAA] can be in one of three different states: It can be ergine in the chair or boat conformation, or it can be isoergine in the chair conformation. And in many chemical and physical situations, the three forms are constantly converting from one form to the others, and achieving a typical equilibrium distribution.
We therefore must consider that in an ergot preparation made according to our suggested method — and in morning glory seeds as prepared by Mesoamerican shamans — we are not dealing with pure ergine or pure isoergine. Both pure compounds have been tested and found wanting by some investigators, including Dr. Hofmann.
I would like to suggest, therefore, that the equilibrium mixture of ergine and isoergine (with ergine also in equilibrium between its two conformations) may actually be the true psychoactive of the kykeon and ololiuhqui. Since ergine spontaneously changes to isoergine when in solution, over a period of an hour or more, any process to partially hydrolyze the alkaloids of ergot, such as our proposed recipe — or even the Aztec shamans' procedure for extracting ololiuhqui — should result in an equilibrium mixture of the three forms.
Taking either ergine or isoergine as a pure compound, however, may not result in the equilibrium mixture arriving at brain receptors. The equilibrium reaction takes some time to occur, perhaps an hour or more, and is brought about most effectively by basic conditions — and neither in the stomach nor in the blood do we find sufficiently basic conditions for the equilibrium to readily establish itself within a short time period.
Concerning the psychoactivity of ergine/isoergine mixtures, I have long had great confidence that extracts of morning glory seed, and by analogy a partial hydrolysis preparation made from ergot, could be quite powerfully psychoactive. In the late 1960s, when I started my research on these matters, I went to Mexico to experiment with morning glory seeds. To begin, I extracted several kilos of seeds using a simple process, purifying an alcoholic extract between organic solvents in the alternating presence of aqueous solutions of ammonia and tartaric acid.
After a couple days' work, I obtained a nearly colorless syrup that exhibited the bright-blue fluorescence typical of active lysergic acid compounds. A few milligrams of this syrup, taken in a capsule, produced one of the most powerful psychedelic experiences I had known — by then I had already taken large doses of LSD several times as well as a few other notorious psychedelic agents.
It has ever since been a mystery to me why ergine should be such a fickle psychedelic, failing with some trials yet succeeding in others. My explanation — perhaps not entirely satisfactory, I admit — is that my extraction procedure allowed the equilibration of the original extracted ergine to the three ergine variants, and it was this mixture that was so effective. We know, of course, that sometimes a mixture of two or more drugs can be more effective than any single component of the mixture alone.
A question kept popping up in my mind, however: Why shouldn't ergine itself be reliably psychoactive? It is a close relative of LSD and its cousin, the dimethylamide, both of which are undeniably and strongly psychedelic. Perhaps this flipping of ring D between chair and boat conformations made ergine less available to their target brain receptors or even prevented ergine from remaining at receptors known to be affected by LSD.
There are other examples of drugs whose receptor affinity is limited by such conformation change, so it seems a good bet that this might be the case with ergine as well.
LSD, having ethyl groups and not hydrogens on the amide nitrogen, cannot form the hydrogen bond which stabilizes the boat form of the D-ring as in ergine, or which stabilizes isoergine to a unique conformation, so LSD remains exclusively in the chair form, and this might help to explain its extraordinary potency.
An additional consideration about the psychoactivity of LSD compared to that of ergine is also significant. It is, in fact, the stabilization of isoergine in the boat conformation by the hydrogen bond that affects the equilibrium concentrations of the two epimers, ergine vs. isoergine. This will depend on the particular solvent, of course, but in water or in the body, we should expect that an approximately 50/50 mixture of ergine and isoergine will exist. But since there is no hydrogen bonding possible with LSD to stabilize its iso form, the eqilibrium concentration of LSD vs. iso-LSD is far from 50/50. In fact, LSD in water solution or the body is 88% in the active epimer and only 12% iso-LSD. In addition, it takes a very long time - up to a week - for pure active LSD to epimerize to that equilibrium concentration, so that a dose of pure active LSD would arrive in the brain intact, with essentially no conversion to its epimer, iso-LSD.
The Mythology and Chemistry of the Eleusinian Mysteries (lecture given at the 2006 LSD symposium in Basel).
https://www.youtube.com/watch?v=uwfkJkvbR-I&start=3448
Webster starts talking about pharmacodynamics at 54:06 and focuses on LAA at 57:28.
Webster's hypothesis is based on the following study:
Ergoline derivatives—VIII: Configuration and conformation of lysergamides and dihydrolysergamides. L. Bernardi, W. Barbieri, Tetrahedron, Volume 21, Issue 9, 1965, Pages 2539-2551, ISSN 0040-4020, 10.1016/S0040-4020(01)93909-2
Peter Webster left LAH out of his equilibrium hypothesis, probably because he wasn't aware that fresh morning glory seeds contain predominantly LAH (see the last quote in the first reference, above), so I emailed him and asked him if perhaps LAH metabolized into this special equilibrium. Webster told me that he didn't think LAH, alone, would create the equilibirum, but that it could be an important contributor.
I should also point out that a recent study that David Nichols participated in examined the conformations of several ergoamides, including LAA:
First, the key amide side chain of LSD—the group that distinguishes it from the far less hallucinogenic lysergamide (LSA)—adopts a constrained conformation in the binding site that cannot exchange readily with alternative conformational states. This conformation, and by extension the contacts made, is crucial for LSD’s actions, and close analogs that cannot adopt it are much less active in vivo.
Crystal Structure of an LSD-Bound Human Serotonin Receptor. Wacker D, Wang S, McCorvy JD, Betz RM, Venkatakrishnan AJ, Levit A, Lansu K, Schools ZL, Che T, Nichols DE, Shoichet BK, Dror RO, Roth BL. Cell. 2017 Jan 26;168(3):377-389.e12. doi: 10.1016/j.cell.2016.12.033. PMID: 28129538; PMCID: PMC5289311
The fungus Metarhizium brunneum produces ergot alkaloids of the lysergic acid amide class, most abundantly lysergic acid α-hydroxyethylamide (LAH).
...the plant root symbiont and insect pathogen Metarhizium brunneum produces ergonovine and LAH, with the concentration of LAH dwarfing that of ergonovine by approximately 200 to 1[5, 6].
Contribution of a novel gene to lysergic acid amide synthesis in Metarhizium brunneum. Britton, K.N., Steen, C.R., Davis, K.A., Sampson, J.K., Panaccione, D.G. BMC Res Notes 15, 183 (2022). doi: 10.1186/s13104-022-06068-2
Isolates of M. brunneum and M. anisopliae accumulated the lysergic acid amides lysergic acid α-hydroxyethyl amide, ergine, and ergonovine on sucrose-yeast extract agar but not on two other tested media.
Metarhizium spp. produce ergot alkaloids in culture in an isolate-specific manner. Three of 10 tested isolates of M. brunneum accumulated the ergot alkaloids lysergic acid α-hydroxyethylamide (LAH) and its degradation product ergine, as well as lesser quantities of ergonovine, when grown on sucrose-yeast extract agar (SYE) (Fig. 3A; Table 1). These alkaloids were identified by their fluorescence properties and retention times relative to an authentic standard (for ergonovine) or standards prepared from Periglandula sp.-infected Ipomoea leptophylla (LAH and ergine) (39).
The accumulation of ergot alkaloids in M. brunneum depended on the culture medium on which the fungus was grown; isolates of M. brunneum produced ergot alkaloids on SYE but not on corn meal agar or malt extract agar.
All ergot alkaloid-positive species of Metarhizium produced lysergic acid amides: most abundantly LAH (along with its hydrolysis product ergine) and lesser quantities of ergonovine. LAH occurs in four stereoisomers as documented in previous work with Claviceps pasapali (42).
Interestingly, M. brunneum ARSEF 9354 secreted the vast majority of its ergot alkaloids into the culture medium. In this way it differs from other ergot alkaloid-producing fungi, such as N. fumigata and Claviceps purpurea, which retain much of their ergot alkaloids in their mycelia, complicating extraction and purification.
Note: This is a wild strain of M. brunneum, not a GMO.
Several additional Metarhizium species, including M. acridum (two isolates), M. album (three isolates), M. anisopliae (four isolates), M. flavoviride (one isolate), M. guizhouense (two isolates), M. majus (two isolates), M. pingshaense (one isolate), and M. robertsii (two isolates), were tested for accumulation of ergot alkaloids on SYE. Among these additional isolates, only M. anisopliae ARSEF 7426 accumulated detectable ergot alkaloids on SYE when analyzed by HPLC with fluorescence detection (Table 1).
Several Metarhizium Species Produce Ergot Alkaloids in a Condition-Specific Manner. Leadmon CE, Sampson JK, Maust MD, Macias AM, Rehner SA, Kasson MT, Panaccione DG. Appl Environ Microbiol. 2020 Jul 2;86(14):e00373-20. doi: 10.1128/AEM.00373-20
Further Reading: Metarhizium: jack of all trades, master of many. St. Leger RJ, Wang JB. Open Biology, Dec 9, 2020. doi: 10.1098/rsob.200307
1. LAH stability info
2. https://www.reddit.com/r/LSA/commen.../?utm_source=share&utm_medium=web2x&context=3 (see last quote)
3. So, we have the possibility that a molecule of ergine [LAA] can be in one of three different states: It can be ergine in the chair or boat conformation, or it can be isoergine in the chair conformation. And in many chemical and physical situations, the three forms are constantly converting from one form to the others, and achieving a typical equilibrium distribution.
We therefore must consider that in an ergot preparation made according to our suggested method — and in morning glory seeds as prepared by Mesoamerican shamans — we are not dealing with pure ergine or pure isoergine. Both pure compounds have been tested and found wanting by some investigators, including Dr. Hofmann.
I would like to suggest, therefore, that the equilibrium mixture of ergine and isoergine (with ergine also in equilibrium between its two conformations) may actually be the true psychoactive of the kykeon and ololiuhqui. Since ergine spontaneously changes to isoergine when in solution, over a period of an hour or more, any process to partially hydrolyze the alkaloids of ergot, such as our proposed recipe — or even the Aztec shamans' procedure for extracting ololiuhqui — should result in an equilibrium mixture of the three forms.
Taking either ergine or isoergine as a pure compound, however, may not result in the equilibrium mixture arriving at brain receptors. The equilibrium reaction takes some time to occur, perhaps an hour or more, and is brought about most effectively by basic conditions — and neither in the stomach nor in the blood do we find sufficiently basic conditions for the equilibrium to readily establish itself within a short time period.
Concerning the psychoactivity of ergine/isoergine mixtures, I have long had great confidence that extracts of morning glory seed, and by analogy a partial hydrolysis preparation made from ergot, could be quite powerfully psychoactive. In the late 1960s, when I started my research on these matters, I went to Mexico to experiment with morning glory seeds. To begin, I extracted several kilos of seeds using a simple process, purifying an alcoholic extract between organic solvents in the alternating presence of aqueous solutions of ammonia and tartaric acid.
After a couple days' work, I obtained a nearly colorless syrup that exhibited the bright-blue fluorescence typical of active lysergic acid compounds. A few milligrams of this syrup, taken in a capsule, produced one of the most powerful psychedelic experiences I had known — by then I had already taken large doses of LSD several times as well as a few other notorious psychedelic agents.
It has ever since been a mystery to me why ergine should be such a fickle psychedelic, failing with some trials yet succeeding in others. My explanation — perhaps not entirely satisfactory, I admit — is that my extraction procedure allowed the equilibration of the original extracted ergine to the three ergine variants, and it was this mixture that was so effective. We know, of course, that sometimes a mixture of two or more drugs can be more effective than any single component of the mixture alone.
A question kept popping up in my mind, however: Why shouldn't ergine itself be reliably psychoactive? It is a close relative of LSD and its cousin, the dimethylamide, both of which are undeniably and strongly psychedelic. Perhaps this flipping of ring D between chair and boat conformations made ergine less available to their target brain receptors or even prevented ergine from remaining at receptors known to be affected by LSD.
There are other examples of drugs whose receptor affinity is limited by such conformation change, so it seems a good bet that this might be the case with ergine as well.
LSD, having ethyl groups and not hydrogens on the amide nitrogen, cannot form the hydrogen bond which stabilizes the boat form of the D-ring as in ergine, or which stabilizes isoergine to a unique conformation, so LSD remains exclusively in the chair form, and this might help to explain its extraordinary potency.
An additional consideration about the psychoactivity of LSD compared to that of ergine is also significant. It is, in fact, the stabilization of isoergine in the boat conformation by the hydrogen bond that affects the equilibrium concentrations of the two epimers, ergine vs. isoergine. This will depend on the particular solvent, of course, but in water or in the body, we should expect that an approximately 50/50 mixture of ergine and isoergine will exist. But since there is no hydrogen bonding possible with LSD to stabilize its iso form, the eqilibrium concentration of LSD vs. iso-LSD is far from 50/50. In fact, LSD in water solution or the body is 88% in the active epimer and only 12% iso-LSD. In addition, it takes a very long time - up to a week - for pure active LSD to epimerize to that equilibrium concentration, so that a dose of pure active LSD would arrive in the brain intact, with essentially no conversion to its epimer, iso-LSD.
The Mythology and Chemistry of the Eleusinian Mysteries (lecture given at the 2006 LSD symposium in Basel).
https://www.youtube.com/watch?v=uwfkJkvbR-I&start=3448
Webster starts talking about pharmacodynamics at 54:06 and focuses on LAA at 57:28.
Webster's hypothesis is based on the following study:
Ergoline derivatives—VIII: Configuration and conformation of lysergamides and dihydrolysergamides. L. Bernardi, W. Barbieri, Tetrahedron, Volume 21, Issue 9, 1965, Pages 2539-2551, ISSN 0040-4020, 10.1016/S0040-4020(01)93909-2
Peter Webster left LAH out of his equilibrium hypothesis, probably because he wasn't aware that fresh morning glory seeds contain predominantly LAH (see the last quote in the first reference, above), so I emailed him and asked him if perhaps LAH metabolized into this special equilibrium. Webster told me that he didn't think LAH, alone, would create the equilibirum, but that it could be an important contributor.
I should also point out that a recent study that David Nichols participated in examined the conformations of several ergoamides, including LAA:
First, the key amide side chain of LSD—the group that distinguishes it from the far less hallucinogenic lysergamide (LSA)—adopts a constrained conformation in the binding site that cannot exchange readily with alternative conformational states. This conformation, and by extension the contacts made, is crucial for LSD’s actions, and close analogs that cannot adopt it are much less active in vivo.
Crystal Structure of an LSD-Bound Human Serotonin Receptor. Wacker D, Wang S, McCorvy JD, Betz RM, Venkatakrishnan AJ, Levit A, Lansu K, Schools ZL, Che T, Nichols DE, Shoichet BK, Dror RO, Roth BL. Cell. 2017 Jan 26;168(3):377-389.e12. doi: 10.1016/j.cell.2016.12.033. PMID: 28129538; PMCID: PMC5289311
- Status
- Not open for further replies.