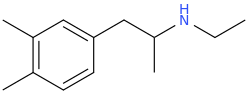
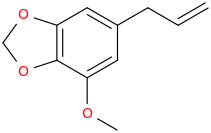
-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Ketamine salts solubility
- Thread starter fastandbulbous
- Start date
- Status
- Not open for further replies.
AlsoTapered
Bluelighter
- Joined
- Apr 1, 2023
- Messages
- 3,076
AlsoTapered
Bluelighter
- Joined
- Apr 1, 2023
- Messages
- 3,076
Yes, because it won't be the same procedure as fenfluramine synthesis in the slightest manner. One simply adjusts for MW. Even the solubilities of the precursors and intermediates only deviates by a gnats-chuff. Why not go mad and make the chiral compound?
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,498
The fenfluramine synthesis is rather odd. I would take a Shulgin-esque approach for ELEANOR.
Why not make the chiral compound? That would give the chiral compound. These next 2 would be the bromopropionate Friedels-Kraft route.

JUNE_BUG
1-(4-methylphenyl)-1-oxo-2-ethylaminopropane
Basically it's just N-ethyl-4-MMC / mephedrone.
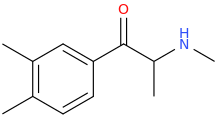
XYLODRONE
1-(3,4-dimethylphenyl)-1-oxo-2-methylaminopropane
Looks good a moi.
Why not make the chiral compound? That would give the chiral compound. These next 2 would be the bromopropionate Friedels-Kraft route.

JUNE_BUG
1-(4-methylphenyl)-1-oxo-2-ethylaminopropane
Basically it's just N-ethyl-4-MMC / mephedrone.

XYLODRONE
1-(3,4-dimethylphenyl)-1-oxo-2-methylaminopropane
Looks good a moi.
Last edited:
AlsoTapered
Bluelighter
- Joined
- Apr 1, 2023
- Messages
- 3,076
Friedel–Crafts acylation. Fine if you think working with bromine is a good move. It's cheap crap made my semi-skilled Chinese.
4-methylethcathinone (4-MEC) is already known as NRG-2.
3,4-dimethylethcathinone (3,4-MEC) is already known as NRG-7.
Wow, that took a 30 second search of Wikipedia...
4-methylethcathinone (4-MEC) is already known as NRG-2.
3,4-dimethylethcathinone (3,4-MEC) is already known as NRG-7.
Wow, that took a 30 second search of Wikipedia...
AlsoTapered
Bluelighter
- Joined
- Apr 1, 2023
- Messages
- 3,076
OK - how are you making the above? I mean what's your commercial precursors?
sekio
Bluelight Crew
- Joined
- Sep 14, 2009
- Messages
- 21,994
He is the kind of chemist I like and respect the least - the infamous paper chemist. A paper chemist can know anything from a little to a lot about chemistry, and might even be a competent researcher or teacher. But when it comes to any actual practical chemistry they usually lack any experience, and left to their own devices in a real lab they are more likely to make a tremendous mess, damage equipment, or even cause injury than achieve even partial results attempting to carry out any sort of synthesis unsupervised.OK - how are you making the above?
I met one before, a new hire at my job. He had a Ph.D and all sorts of accolades and awards. We asked him to make a few hundred grams of a known compound that had a simple synthesis from circa 1900 using common and cheap reagents, and a trivial purification method. A few weeks later he had managed to start a fire attempting a synthesis, but eventually produced perhaps 0.5g of compound or so.
The final straw, I gave him a complex terpene mixture to seperate with column chromatography. He dutifully did so, but neglected to actually analyse the fractions collected. When he gave me back a tray of 50 vials or so, I was dismayed to see that the first 30 contained only solvent and the next 20 - the very least polar and first eluting compound. He had simply collected 50 fractions, tested none of them in any way, and based on nothing at all, decided that was good enough.
He did not stay employed for long after that one...
AlsoTapered
Bluelighter
- Joined
- Apr 1, 2023
- Messages
- 3,076
Maybe you ought to invest in some appropriate software? Older versions of ChemOffice turn up on Ebay from time to time for about £100.
Newer versions are all downloads and are designed to send you to Elsevier to BUY the references whereas those who know simply use Sci Hub.
If nothing else, it provides pretty pictures and provides for things like GMEC calculations so you can see what the chemical will look like 'in nature' although to be fair, I know a lot of compounds bind in a near-GMEC conformation.
Newer versions are all downloads and are designed to send you to Elsevier to BUY the references whereas those who know simply use Sci Hub.
If nothing else, it provides pretty pictures and provides for things like GMEC calculations so you can see what the chemical will look like 'in nature' although to be fair, I know a lot of compounds bind in a near-GMEC conformation.
AlsoTapered
Bluelighter
- Joined
- Apr 1, 2023
- Messages
- 3,076
Now of course we went a step further an carried a proper human study. Pyeyzolam (selective a5) and Zolpidem (selective a1) strongly suggest that all of the positive subjective effects of alcohol are mediated by the a5 subunit whereas the negative effects are mediated by the a1 subunit. Anyone familiar with Zoplidem will recognise that it does has so nasty psychological side-effects such as loss of executive control, retrograde amnesia, mood lability and so forth.
The few who have sampled pyeyzolam will recognize that huge difference in subjective effects. One can get roughly 2 bottles of wine drunk inside 30 minutes and yet not fall asleep not think or do anything inappropriate, stupid or harmful AND the euphoria is cleaner. I know memory-loss was not an issue. Of course, it MAY have some re-enforcing effects but it totally stopped cravings in alcohol dependant subjects.
So don't expect to see it as a street drug, expect it as a medicine to treat alcoholism and maybe, in the fullness of time, as an alcohol alternative.
- Joined
- Jul 22, 2018
- Messages
- 639
Supposedly, there is at least one highly valued claviceps paspali mutant that produces direct LA. Was also told it yields 3g of LA per liter of culture every 10 days.The problem is, ergot doesn't make LSA.
I actually have a bioreactor but never tried to culture anything yet.
It cant be that difficult? Most natural products just require specific extraction and purification protocols. I employ a ghetto version of flash chromatography similar to CRC in cannabis you just gotta find where your target molecule elutes.. but alot of the pigments are relatively polar and so will stick to the media.How are you going to isolate workable amounts of LSA anyway? Attempting to do chemistry on crude mixtures is a recipe for frustration.
Haven't tried it with morning glories, but it does wonders on other natural products
AlsoTapered
Bluelighter
- Joined
- Apr 1, 2023
- Messages
- 3,076
If I may say so, being high only makes a minority of people jealous. Nobody I KNOW would be jealous.
Don't know of the second item.
Start with something legal and simple. p-F ethylamphetamine isn't controlled in most places, p-F P2P is commercially available and adapting this patent is quite possibly the single, simplest route. https://patents.google.com/patent/US4536518A/en
Don't know of the second item.
Start with something legal and simple. p-F ethylamphetamine isn't controlled in most places, p-F P2P is commercially available and adapting this patent is quite possibly the single, simplest route. https://patents.google.com/patent/US4536518A/en
Last edited:
AlsoTapered
Bluelighter
- Joined
- Apr 1, 2023
- Messages
- 3,076
| ID ↕ Time add. ↕ Title ↕ Series ↕ | Author(s) ↕ | Publisher ↕ | Year ↕ | Pages | Language | |
|---|---|---|---|---|---|---|
| Chemischer Informationsdienst vol. 10 iss. 18 ChemInform Abstract: STUDIES ON 1-SUBSTITUTED 4-(1,2-DIPHENYLETHYL)PIPERAZINE DERIVATIVES AND THEIR ANALGESIC ACTIVITIES. 2. STRUCTURE-ACTIVITY RELATIONSHIPS OF 1-CYCLOALKYL-4-(1,2-DIPHENYLETHYL)PIPERAZINES DOI: 10.1002/chin.197918274 a a | NATSUKA, K.; NAKAMURA, H.; NEGORO, T.; UNO, H.; NISHIMURA, H. | 1979 May 01 | ||||
| Journal of Medicinal Chemistry vol. 21 iss. 12 pp.1265—1269 Studies on 1-substituted 4-(1,2-diphenylethyl)piperazine derivatives and their analgesic activities. 2. Structure-activity relationships of 1-cycloalkyl-4-(1,2-diphenylethyl)piperazines DOI: 10.1021/jm00210a017 a a | Natsuka, Kagayaki; Nakamura, Hideo; Negoro, Toshiyuki; Uno, Hitoshi; Nishimura, Haruki | 1978 December | English |
- Status
- Not open for further replies.