Fertile
Bluelighter
- Joined
- Mar 31, 2022
- Messages
- 1,627
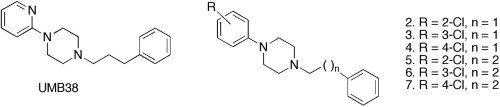
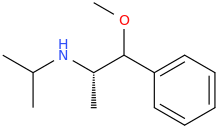
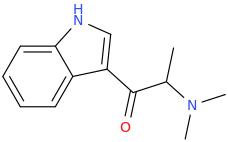
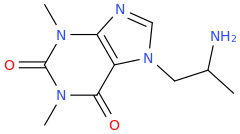
So it's phenibut but with the alkyl chain altered to overlay valeric acid? Well the former directly binds to GABAb, the latter is a GABAa PAM.
I would be interested to know your thinking as you designed it. Are their receptor models for the binding sites? Do you have a facile synthesis?
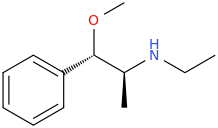
Oh, and it's chiral so which isomer do you think will be active?
I would be interested to know your thinking as you designed it. Are their receptor models for the binding sites? Do you have a facile synthesis?
Oh, and it's chiral so which isomer do you think will be active?