sekio
Bluelight Crew
- Joined
- Sep 14, 2009
- Messages
- 21,994
> If you gave me a listing of bond lengths I could provide rough pKa on the fly. You know, with a pen and a bar napkin.
I dunno how you'd do that, but OK:
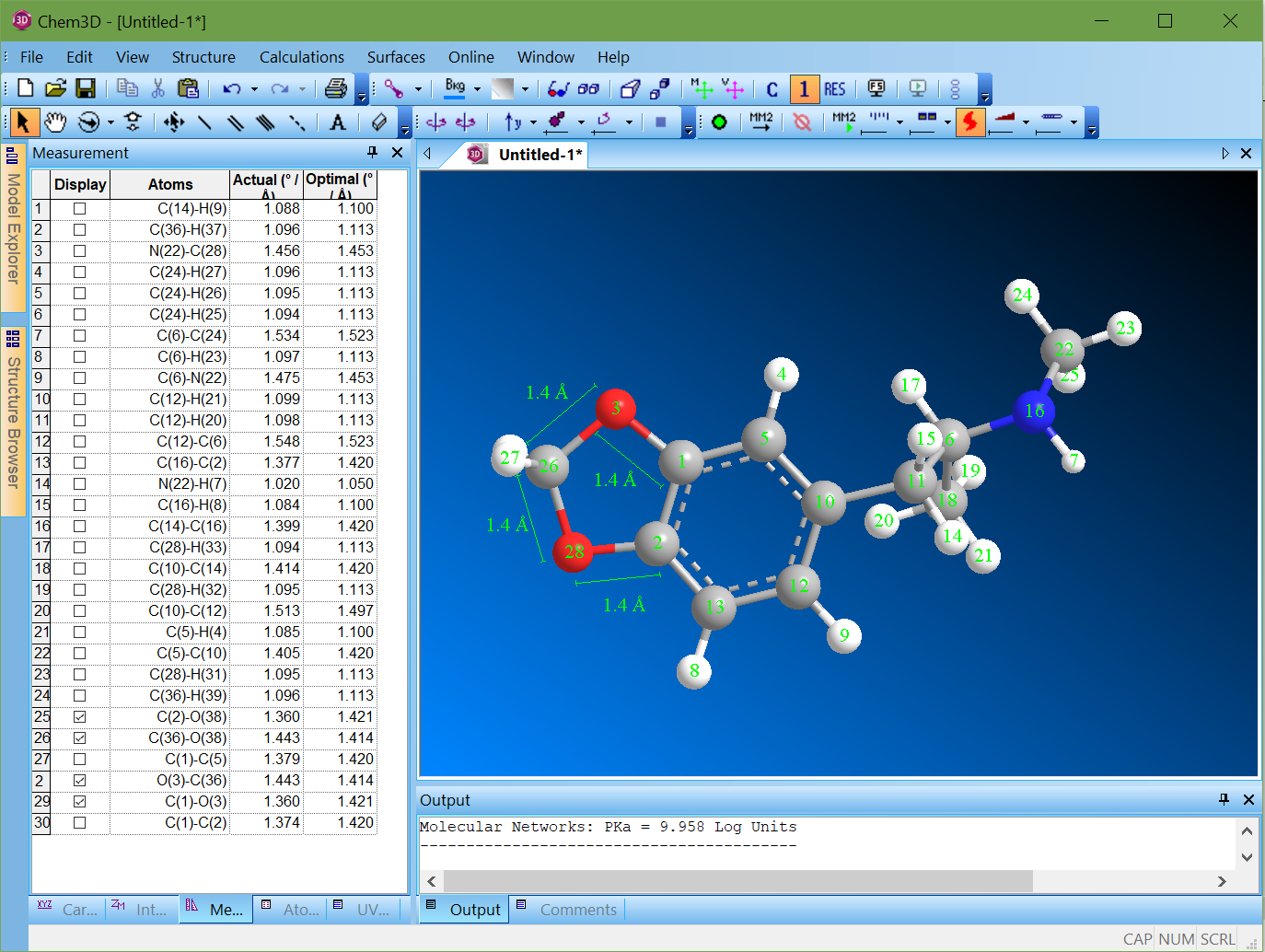
> So in other words the implied pKb of the diene bonds is to low to present a reasonable reaction target in the stomach. Got it. If you'd wanted to save ink, a listing of the pkB for the structure would have accomplished the same thing.
Diene bonds? There is no diene in MDMA. The aromatic phenyl ring has delocalized bonds that are by convention drawn as alternating single and double bonds, but in reality they are all the same length.
The pKa of the conjugate acid of the molecule is going to be determined by its most easily deprotonated group which will be the amine. The predicted pKa of benzodioxole's conjugate acid is like -4.7, meaning the ring is basically never going to accept a proton, especially compared to the basic nitrogen in an MDA analogue.
Safrole is stable enough to react with hydrogen bromide without opening the MD ring [ref], in fact that was how MDMA was originally made.
> I'm an outsider to your field but I'm not sure that someone in your field would document a thing just because it happened.
MDMA is not a new substance, and if it were acid sensitive, that would be a notable enough observation that at least someone would have noticed it. Also, it is successfully administered in acidic drinks like cola, juice, etc.
> At any rate MDMA is at least stable enough with HCL that they can form a salt together, right?
Exactly. It can form a salt from HCl gas or any source. You can form MDMA.HCl even by adding liquid 37% concentrated HCl to the freebase and boil the water off or add a countersolvent to crystallize it.
> Typically when I set out to change someone's mind, I ask them to see what I would accept as proof rather than tell them what they should accept as proof. Unless you'd take the Bible as proof because I say you should? People keep telling me not to do that.
Among scientists worldwide, the scientific literature is usually considered to be reliable proof. Especially from peer-reviewed, well-established journals.
I dunno how you'd do that, but OK:

> So in other words the implied pKb of the diene bonds is to low to present a reasonable reaction target in the stomach. Got it. If you'd wanted to save ink, a listing of the pkB for the structure would have accomplished the same thing.
Diene bonds? There is no diene in MDMA. The aromatic phenyl ring has delocalized bonds that are by convention drawn as alternating single and double bonds, but in reality they are all the same length.
The pKa of the conjugate acid of the molecule is going to be determined by its most easily deprotonated group which will be the amine. The predicted pKa of benzodioxole's conjugate acid is like -4.7, meaning the ring is basically never going to accept a proton, especially compared to the basic nitrogen in an MDA analogue.
Safrole is stable enough to react with hydrogen bromide without opening the MD ring [ref], in fact that was how MDMA was originally made.
> I'm an outsider to your field but I'm not sure that someone in your field would document a thing just because it happened.
MDMA is not a new substance, and if it were acid sensitive, that would be a notable enough observation that at least someone would have noticed it. Also, it is successfully administered in acidic drinks like cola, juice, etc.
> At any rate MDMA is at least stable enough with HCL that they can form a salt together, right?
Exactly. It can form a salt from HCl gas or any source. You can form MDMA.HCl even by adding liquid 37% concentrated HCl to the freebase and boil the water off or add a countersolvent to crystallize it.
> Typically when I set out to change someone's mind, I ask them to see what I would accept as proof rather than tell them what they should accept as proof. Unless you'd take the Bible as proof because I say you should? People keep telling me not to do that.
Among scientists worldwide, the scientific literature is usually considered to be reliable proof. Especially from peer-reviewed, well-established journals.
Last edited:

