Soulfake
Bluelighter
- Joined
- Aug 12, 2010
- Messages
- 160
I've been thinking about what would happen if you tried to isomerize H4-CBD the way you would regular CBD. One would expect to simply obtain HHC and various variants thereof, depending on the reaction details, instead of d8/9-THC.
Then I took a closer look at the molecule and noticed that there is a difference other than the ring being hydrogenated. A carbon atom is missing from the position required for isomerization. Is this a problem? I hope some knowledgeable people can help me, I'm still a layman after so many years of "research" ^^'
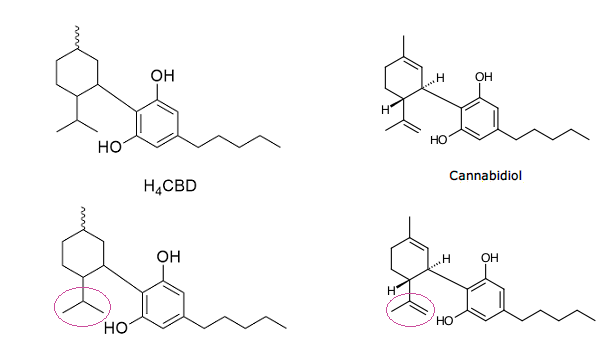
Then I took a closer look at the molecule and noticed that there is a difference other than the ring being hydrogenated. A carbon atom is missing from the position required for isomerization. Is this a problem? I hope some knowledgeable people can help me, I'm still a layman after so many years of "research" ^^'

