Serhat
Bluelighter
- Joined
- Jan 6, 2023
- Messages
- 704
Hi Skorpio, I appreciate your detailed response and the points you've raised. I agree with you on the limitations of ChatGPT and the importance of relying on primary evidence and specialized databases.ChatGPT is good for a bunch of things. It can rephrase writing and it is good at defining syntax for computer languages, but it is not reliable with facts. If you search journal articles, it will rework your question into paper titles, with DOIs which direct to random papers, as it knows the format of a paper reference and the makeup of a DOI. If you ask it to work through algebra, it will make mistakes and include things form outside the problem. If you ask it pharmacology questions, it will answer in a way that looks accurate, but the definate values and statements are not based in reality.
The different logPs from drugbank are due to differences in algorithms used. They are still computing the same input data. Their spread is instrument error, not a random number.
If something has a charge, it isn't crossing a lipid membrane, unless it is functioning like a detergant (and detergants at their critical micelle concentration would be lethal because of their dissolution of membranes). Lipophilicity can be altered by the balance of polar and nonpolar constituents of a molecule, but a charge is going to be a stronger force than anyother features of a molecule. If a peptide is charged, it is only going to be taken up by transporters (which I believe I stated in my original post).
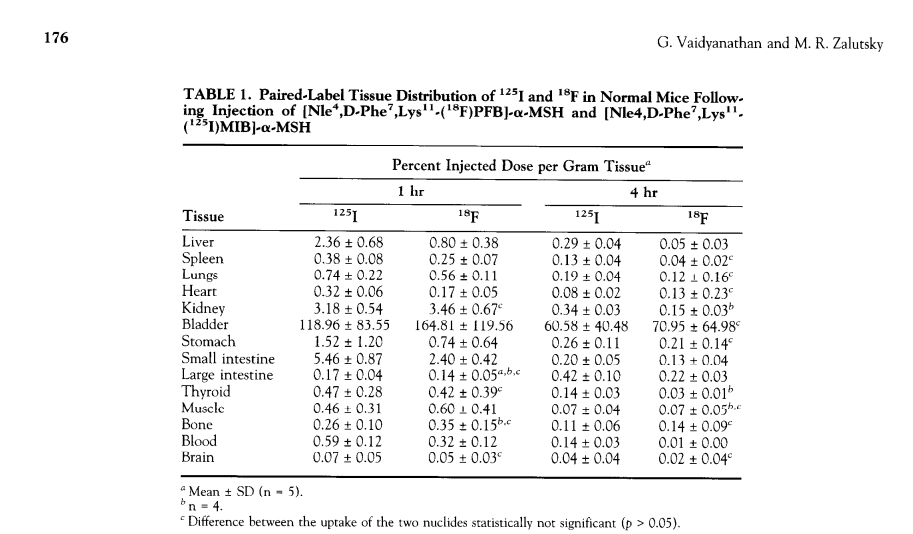
Also if you've got a single paper with primary evidence, that is enough for me with regards to the brain penetration of afamelanotide. One of the links was to this thread.
The paper below talks about how melanotan I analogs were developed to provide blood brain barrier penetration.
Novel approaches to the design of bioavailable melanocortins:
I found your insights on the role of charge in membrane permeability to be quite enlightening. Your emphasis on the deterministic role of charge in a molecule's ability to cross lipid membranes is well-taken. However, I recently came across an article that adds a layer of complexity to this discussion.
The article, titled "Novel approaches to the design of bioavailable melanotropins" (link), delves into the intricacies of melanocortin receptors and their ligands. It discusses various strategies to improve the bioavailability of melanotropins, including cyclization and N-methylation. While the article is comprehensive, it does have some limitations that warrant discussion.
Firstly, the article seems to focus predominantly on the structural modifications of melanotropins to improve their bioavailability, but it doesn't sufficiently address the physiological transport mechanisms that would allow these modified peptides to cross the blood-brain barrier or other lipid membranes. This leaves a gap in our understanding of how these peptides interact with cellular transporters, which you rightly pointed out as being crucial for charged molecules.
Secondly, the article discusses the potential for biased signaling events on melanocortin receptors but doesn't provide empirical data to substantiate this claim. This raises questions about the physiological relevance of these signaling pathways and how they might interact with the charge and lipophilicity of the peptides.
Lastly, while the article does mention the potential for cyclotides and constrained tetrapeptides to improve oral bioavailability, it doesn't delve into the pharmacokinetics of these molecules. Understanding how these peptides are absorbed, distributed, metabolized, and excreted is crucial for assessing their potential as drug candidates.
Given these considerations, I would argue that while charge is undoubtedly a significant factor in membrane permeability, the interaction between peptides and cellular transporters, as well as the potential for alternative signaling pathways, adds a layer of complexity that shouldn't be overlooked.
I would be interested to hear your thoughts on these points.