-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Ketamine salts solubility
- Thread starter fastandbulbous
- Start date
- Status
- Not open for further replies.
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,466
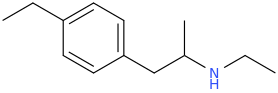
The N being just one C away from the SO2 functional group is a no go, right?
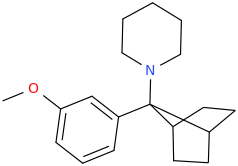
THE_INCREDIBLE_HULK
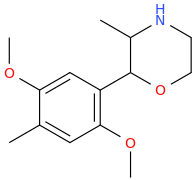
2-(3-methoxyphenyl)-2-(1-piperidinyl)-bicyclo[2.1.2]heptane
You're right. This one might be impossible, too.
Anyway I've been drawing these molecules all day, but the most promising are probably TALISHA and FENYL_FEINDING and their 3,4-MDO and 4-MeO analogues. At least, I feel as though they could definitely be made. But, then again, that might just be me being naive.

THE_INCREDIBLE_HULK
2-(3-methoxyphenyl)-2-(1-piperidinyl)-bicyclo[2.1.2]heptane
You're right. This one might be impossible, too.
Anyway I've been drawing these molecules all day, but the most promising are probably TALISHA and FENYL_FEINDING and their 3,4-MDO and 4-MeO analogues. At least, I feel as though they could definitely be made. But, then again, that might just be me being naive.
Last edited:
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,466
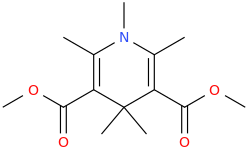
SWEET_CAROLINE
2,6-dimethyl-3,5-bis-carbomethoxy-N-methyl-1-aza-4,4-dimethyl-cyclohex-2,5-diene
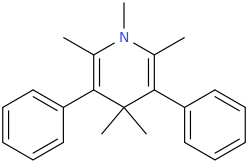
AROMATICITY
2,6-dimethyl-3,5-bis-phenyl-N-methyl-1-aza-4,4-dimethyl-cyclohex-2,5-diene
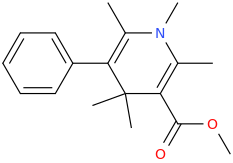
HUCKEL'S_RULE
2,6-dimethyl-3-phenyl-5-carbomethoxy-N-methyl-1-aza-4,4-dimethyl-cyclohex-2,5-diene
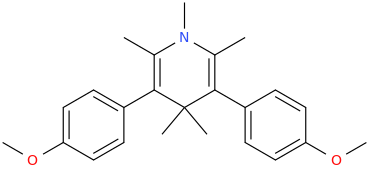
NUMINOUS
2,6-dimethyl-3,5-bis(4-methoxyphenyl)-N-methyl-1-aza-4,4-dimethyl-cyclohex-2,5-diene
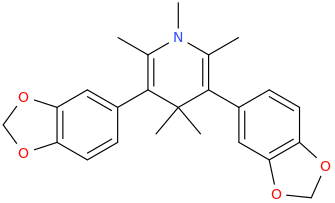
VOLUMINOUS
2,6-dimethyl-3,5-bis(3,4-methylenedioxyphenyl)-N-methyl-1-aza-4,4-dimethyl-cyclohex-2,5-diene
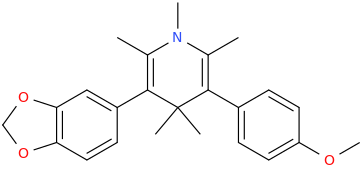
BURNING_MAN
2,6-dimethyl-3-(3,4-methylenedioxyphenyl)-5-(4-methoxyphenyl)-N-methyl-1-aza-4,4-dimethyl-cyclohex-2,5-diene
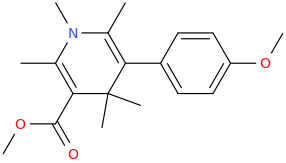
RIGOROUS
2,6-dimethyl-3-carbomethoxy-5-(4-methoxyphenyl)-N-methyl-1-aza-4,4-dimethyl-cyclohex-2,5-diene
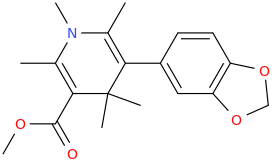
VOCIFEROUS
2,6-dimethyl-3-carbomethoxy-5-(3,4-methylenedioxyphenyl)-N-methyl-1-aza-4,4-dimethyl-cyclohex-2,5-diene
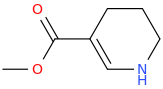
BETELGEUSE
1-aza-3-carbomethoxycyclohex-2-ene
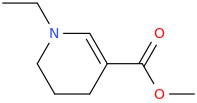
SOUTH_ARECOLINE_CAROLINA
N-ethyl-1-aza-3-carbomethoxycyclohex-2-ene
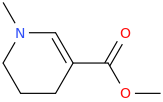
NORTH_ARECOLINE_CAROLINA
N-methyl-1-aza-3-carbomethoxycyclohex-2-ene
Last edited:
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,466
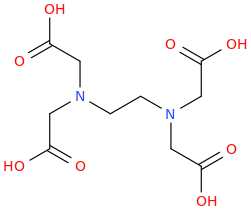
EDTA
EthyleneDiamineTetra-Acetic acid
Using EDTA as the solvent in the Birch Reduction [Li metal; anhydrous NH3 or ammonium nitrate, NH3+NO2] of PPA or ephedrine to amp or methamphetamine makes the reaction work in just 60 seconds aka 1 minute!
Neuroprotection
Bluelighter
- Joined
- Apr 18, 2015
- Messages
- 1,091
as I am blind, I’m not sure if you’re all posting images of the molecules you propose, Plus I’m no expert in chemistry. however, just want to suggest deschlorobupropion. that would be bupropion without the chlorine atom in the molecule. Of course, that chlorine atom would have to be replaced by a hydrogen. does anyone know what the pharmacological properties of this molecule could be?
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,466
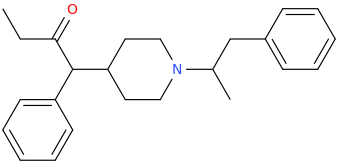
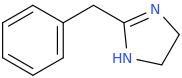
Stronger stimulation but less color enhancement. Probably has to be snorted or injected to give the desired effects, like bupropion. Here, let me post the structure we're talking about:
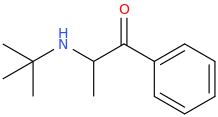
NEUROPROTECTION
N-tert-butyl-1-phenyl-1-oxo-2-aminopropane
There you go, I even named it after your username.

NEUROPROTECTION
N-tert-butyl-1-phenyl-1-oxo-2-aminopropane
There you go, I even named it after your username.
AlsoTapered
Bluelighter
- Joined
- Apr 1, 2023
- Messages
- 3,076
as I am blind, I’m not sure if you’re all posting images of the molecules you propose, Plus I’m no expert in chemistry. however, just want to suggest deschlorobupropion. that would be bupropion without the chlorine atom in the molecule. Of course, that chlorine atom would have to be replaced by a hydrogen. does anyone know what the pharmacological properties of this molecule could be?
Removing that meta chloro moiety will increase the activity of the drug, Specifically it will engender at least some SERT activity and most likely increase reuptake inhibition of all three monoamines.
- Joined
- May 11, 2011
- Messages
- 3,388
This one I'm quite fond of. So many uses.
EDTA
EthyleneDiamineTetra-Acetic acid
Using EDTA as the solvent in the Birch Reduction [Li metal; anhydrous NH3 or ammonium nitrate, NH3+NO2] of PPA or ephedrine to amp or methamphetamine makes the reaction work in just 60 seconds aka 1 minute!
AlsoTapered
Bluelighter
- Joined
- Apr 1, 2023
- Messages
- 3,076
This one I'm quite fond of. So many uses.
It's a chelating agent - so it's often used to treat metal poisoning.
- Joined
- May 11, 2011
- Messages
- 3,388
It also inhibits a bunch of enzymes, especially proteases (pretty much anything with a metal active site), can elute proteins of nickel chromatography resin, can be bacteriostatic, and can stabilize unstable things due to metals.It's a chelating agent - so it's often used to treat metal poisoning.
AlsoTapered
Bluelighter
- Joined
- Apr 1, 2023
- Messages
- 3,076
Birch reductions are a class of reaction. With regards to the use of ETDA, it would appear that it's more generally applicable to the reduction of aromatic rings:

 pubmed.ncbi.nlm.nih.gov
pubmed.ncbi.nlm.nih.gov
So it wouldn't be of much use in the reduction of a benzylic hydroxyl.
I'm quite prepared to read any papers in which ETDA is used for that specific class of Birch reduction.

Scalable Birch reduction with lithium and ethylenediamine in tetrahydrofuran - PubMed
The Birch reduction dearomatizes arenes into 1,4-cyclohexadienes. Despite substantial efforts devoted to avoiding ammonia and cryogenic conditions, the traditional, cumbersome, and dangerous procedure remains the standard. The Benkeser reduction with lithium in ethylenediamine converts arenes to...
So it wouldn't be of much use in the reduction of a benzylic hydroxyl.
I'm quite prepared to read any papers in which ETDA is used for that specific class of Birch reduction.
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,466
AlsoTapered,
You are often right, but this time you are just flat out wrong. I read an article 2 months ago that said exactly what I wrote. I am not going to argue with you or be called wrong about this. I am beginning to wonder if you are an argumentative AI chatbot.
You are often right, but this time you are just flat out wrong. I read an article 2 months ago that said exactly what I wrote. I am not going to argue with you or be called wrong about this. I am beginning to wonder if you are an argumentative AI chatbot.
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,466
This is the closest I could find. It replaces NH3 with ethylenediamine in THF.
Scalable Birch reduction with lithium and ethylenediamine in ... https://www.researchgate.net/public...ithium_and_ethylenediamine_in_tetrahydrofuran
I would substitute EDTA for ethylenediamine if I were a chemist based on what I read the other day but can't find now.
Scalable Birch reduction with lithium and ethylenediamine in ... https://www.researchgate.net/public...ithium_and_ethylenediamine_in_tetrahydrofuran
I would substitute EDTA for ethylenediamine if I were a chemist based on what I read the other day but can't find now.
- Status
- Not open for further replies.