Do you mean burn as in on insufflation? or with respect to attempting vaporization and inhalational delivery, I.e physical pyrolysis?
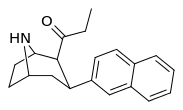
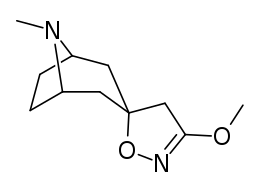
Definitely will keep these in mind. Any papers on lefetamine analog SAR would be appreciated if you have any. I know more about PCP etc. SAR than I do about ket and its analogs, and little on lefetamine specifically. Although its not been one I've looked into deeply, its only by chance and of late that the diphenylacetic acid became available, 30g to play with though, and lefetamine itself has always been an intriguing drug, due to its triple-whammy of psychostimulant, NMDA antagonism and MOR opioidergic effects.
That sounds to me very much like a 'whats not to like' and 'potential winner' right there, with regards to lefetamine.
I've never tried it, not yet at least. But I have tried diphenidine and methoxphenidine, although not ephenidine and I LOVED both, diphenidine especially.
Also, DECREASING duration of effect? unless that expense is to be a cost paid for much improved subjective 'flavour' then why? why would anybody want to do that? I find short-acting dissociatives to be all the more compulsive redosers compared to something with a nice few hours at least (and something really long like very high dose IV memantine, now that hits the spot. i've always wanted to try MK-801, as well as a combination of MK-801 and a small amount of some sort of dopaminergic reuptake inhibitor just because MK-801 is so selective, but, on the whole, I don't think I've ever met an NMDA antagonist I didn't take to like a blowfly takes to a fresh, juicy steaming turd

)
Do I like my NMDA antagonists? yes, yes I do. At recreational doses, one of my favourite classes of all drugs, especially with a little cannabinoid action added from whatever happens to be available; and at what one would probably call therapeutic dosing regimes, then NMDA antagonists seem somehow to 'normalize' something that was mis-configured in my original factory settings. And as an added subjective bonus, if this makes sense, then they almost....its as if they make me 'feel', subjectively speaking, 'more autistic' (I was born classically/kanner's autie. And I like it that way. I find the thought that I might not have been born that way nothing short of a nightmarish proposition if I'm quite honest. Doesn't mean I hate NT people, I don't. Well, not all NT people, and not BECAUSE they are NT, met plenty NT assholes, but thats on an individual basis, although some common factors do often occur as comorbids in their cases. Although I'm not one of the autistic supremacist types who thinks of ours as the 'master race'; not looking for a position as the first ReichsFuhrer of Autia. Although if the post came up for grabs, I'd take it to prevent others doing so because I'd probably do a better job and avoid as much butchery or/and deportations of NTs as humanly and humanely possible (yes, there are people who would at least split up families and deport the NT-born at age 18. I'm not, however, one of them)
NMDA antagonists though do definitely make me feel 'more' autistic. What some (NTs, naturally, the curebie ones in particular, would call 'severely', but I personally, prefer the term 'profoundly', because profound is what to experience being autistic is, in so many ways. There might be downsides, but for me, the upsides overrule those by a long shot, and a profound experience of life is exactly what I have been fortunate enough to be blessed with. I'd not give that away for love, nor money, nor the best damn analytical electronics for my lab that both put together could bring me. Not in a thousand lifetimes. I'd sooner die than wake up tomorrow and find I had been magically cursed to lose my being autie. I don't think I could live without it, having spent all my life with it. I'd not be ME anymore. And its deep, profound, all-encompassing, touching every aspect of life and beautiful

Curebies don't see that of course, they never will. I wish there could be a pill, a shot, depot implant etc. that could allow people to transition from NT to autie or to aspie according to preference for a given time, a week, a month, a year. I could really see such a pharmaceutical temporary cure for NT-ism selling like hot cakes and cold beer to a famine-beset third world country. And if it were something short acting, the fentanyl of autie/aspie-shots/pills/transdermal patches then that'd be like crack cocaine to a crackhead. Once an NT tried one, they'd be back pawning their shoes and possibly even their internal organs (not that one can PAWN an innard as such, thats more of a one-way transaction of course

).....but they'd be back for more, mark my words, given a taste, the NTs would be back in such numbers begging for more that the pharm companies would need to have dedicated facilities for production of just the autism-transition pill, and begging for a permanent NT-ceptive for making sure their kids were too.
I know, I know, it isn't ever likely to happen. But a man can dream, can he not? and in this case of sharing something good with the world, not only the Scrooge McDuck-esque gold coin-filled swimming pools to roll around in and stim with some wall and ceiling mounted lights at the sparkly things.... That would just rock so much.. (and flap, spin, twist and bounce and twirl......

)