pr0d1gy
Bluelighter
- Joined
- May 1, 2009
- Messages
- 547
Substituted dihydropyrimidones (DHM) and their sulfanylidene derivatives are chemicals of biological interest. Generally research is directed towards their possible anti-viral, anti-HIV, anti-fungal and anti-cancer properties.
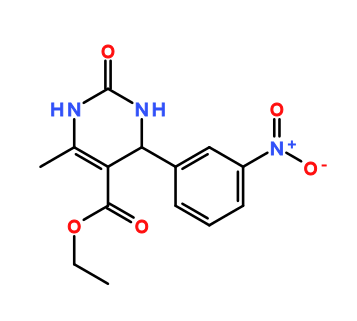
Compounds such as monastral are also known to PAMs for GHB binding sites as well as novel GABA receptor subunits.
The pharmacore somewhat resembles that of barbiturates, benzodiazepines and non-benzodiazepines such as ELB-139. However, DHMs exert their effects very selectively through novel and somewhat unknown subunits.
Some research has been conducted investigating their potential as anti-convulsants. These studies found certain derivatives to be more effective at preventing seizures than diazepam. They also found apparently low toxicity and were generally well tolerate.
I recently experimented with a DHM derivative out of curiosity. The compound doesn’t seem to have a name so I’ll call it JM-II-43B based on the nomenclature used in relevant literature.
I’ll report on my experience with this compound. I’ll also attach literature references and HNMR (because proof is in the pudding) on Monday.
Experience:
+0:00 - Ingested 3 mg of compound as an allergy test, it’s quite insoluble in water but tasteless.
+1:00 - No adverse effects noted, I decide to go all in and ingest 100 mg.
+1:45 - Actually starting to feel something, mostly analgesia. My line of work puts a lot of strain on my back and I also have a headache. Both are disappearing.
+2:00 - Ingest another 200 mg, based on dose corrections for humans I’m well below what’s used in animal trials, still this is reckless.
+2:30 - A clear sense of calm is taking over. Subtle, maybe placebo. My vision also seems to be slightly clearer. It’s night and lights and objects are very vivid in contrast.
+3:00 - Feeling real effects for sure, calm sense of sedation. Not strong but noticeable. My muscles feel less tense. My mood is improved without a doubt. A subtle euphoria somewhat comparable to methaqualone. Not hypnotic like a benzo or barb, not fuzzy headed like methaqualone. It’s hard to describe. Just a general sense of wellbeing and lose of anxiety.
+4:00 – Effects have peaked by now, maybe a bit more sedated but not much. Muscles feel very relaxed and previous aches and pains are gone. I go to bed and fall asleep easily.
Afterwards: Slept a good 12 hours, woke up still generally feeling good. Almost like an anti-depressant effects. I’ve experienced similar effects the day after phenibut but this is more subtle and clear headed. It’s more mental and less physical. I just feel abnormally good in general. This is a surprise because I have a huge number of stressors currently. The dose I took was quite low when corrected from that used in animal tests. Hypothetically, it could have been a lot higher if the toxicity of these compounds in humans correlates to the known animal tests.
It’s probably worth slowly titrating up the dose to see what positive or negative effects appear. It seems possibly that this compound could be quite enjoyable at a higher dose.
So that’s my report. I’m posting this is ADD because this is a novel substance. Based on my totally subjective experience I think DHMs have some potential as recreational substances. Libraries of derivatives exist in research, but said research is almost exclusively related to anti-microbial or anti-carcinogenic properties.
It’s also noteworthy that these substances are incredibly simple to synthesize. Your average graduate student could write “DHMs I Have Known and Loved” in a month or two. I hypothetically may continue to investigate the properties of these substances. I hope that more publications will be made investigating their psychoactive properties, but doubt this will happen.
In conclusion, I think the DHM pharmacore with respect to GABA PAM properties is well worth further investigation. Any comments regarding these compounds or citations to relevant literature would be appreciated!

Compounds such as monastral are also known to PAMs for GHB binding sites as well as novel GABA receptor subunits.
The pharmacore somewhat resembles that of barbiturates, benzodiazepines and non-benzodiazepines such as ELB-139. However, DHMs exert their effects very selectively through novel and somewhat unknown subunits.
Some research has been conducted investigating their potential as anti-convulsants. These studies found certain derivatives to be more effective at preventing seizures than diazepam. They also found apparently low toxicity and were generally well tolerate.
I recently experimented with a DHM derivative out of curiosity. The compound doesn’t seem to have a name so I’ll call it JM-II-43B based on the nomenclature used in relevant literature.
I’ll report on my experience with this compound. I’ll also attach literature references and HNMR (because proof is in the pudding) on Monday.
Experience:
+0:00 - Ingested 3 mg of compound as an allergy test, it’s quite insoluble in water but tasteless.
+1:00 - No adverse effects noted, I decide to go all in and ingest 100 mg.
+1:45 - Actually starting to feel something, mostly analgesia. My line of work puts a lot of strain on my back and I also have a headache. Both are disappearing.
+2:00 - Ingest another 200 mg, based on dose corrections for humans I’m well below what’s used in animal trials, still this is reckless.
+2:30 - A clear sense of calm is taking over. Subtle, maybe placebo. My vision also seems to be slightly clearer. It’s night and lights and objects are very vivid in contrast.
+3:00 - Feeling real effects for sure, calm sense of sedation. Not strong but noticeable. My muscles feel less tense. My mood is improved without a doubt. A subtle euphoria somewhat comparable to methaqualone. Not hypnotic like a benzo or barb, not fuzzy headed like methaqualone. It’s hard to describe. Just a general sense of wellbeing and lose of anxiety.
+4:00 – Effects have peaked by now, maybe a bit more sedated but not much. Muscles feel very relaxed and previous aches and pains are gone. I go to bed and fall asleep easily.
Afterwards: Slept a good 12 hours, woke up still generally feeling good. Almost like an anti-depressant effects. I’ve experienced similar effects the day after phenibut but this is more subtle and clear headed. It’s more mental and less physical. I just feel abnormally good in general. This is a surprise because I have a huge number of stressors currently. The dose I took was quite low when corrected from that used in animal tests. Hypothetically, it could have been a lot higher if the toxicity of these compounds in humans correlates to the known animal tests.
It’s probably worth slowly titrating up the dose to see what positive or negative effects appear. It seems possibly that this compound could be quite enjoyable at a higher dose.
So that’s my report. I’m posting this is ADD because this is a novel substance. Based on my totally subjective experience I think DHMs have some potential as recreational substances. Libraries of derivatives exist in research, but said research is almost exclusively related to anti-microbial or anti-carcinogenic properties.
It’s also noteworthy that these substances are incredibly simple to synthesize. Your average graduate student could write “DHMs I Have Known and Loved” in a month or two. I hypothetically may continue to investigate the properties of these substances. I hope that more publications will be made investigating their psychoactive properties, but doubt this will happen.
In conclusion, I think the DHM pharmacore with respect to GABA PAM properties is well worth further investigation. Any comments regarding these compounds or citations to relevant literature would be appreciated!
