sekio
Bluelight Crew
- Joined
- Sep 14, 2009
- Messages
- 21,994
I think people should know about this. Especially if you are extracting DMT! Here's a site that contains a big database (1000+ entries) of gas chromatograph analyses of many different solvents, fuels, and "ignitable liquids" from different manufacturers.
If you cannot read GC charts, here's a quick rundown: the GC is a machine that seperates compounds according to how volatile they are - you put a mixture into it and over time it will spit out the individual chemical compounds that make up that mixture. Usually, the same compound will always leave the GC at the same time - and it can be hooked up to a mas sspectrometer to get an exact identity.
GC charts are read like a bar graph: Lighter, smaller, more volatile compounds which exit the GC first appear on the left side. As the chart moves to the right, the compounds get progressively less volatile, heavier, and bigger. A bigger "peak" on the chart means more of the compound is present, and a smaller one means only a little was present (the amount of compound is proportional to the peak area, for you math nerds.) There is a bar scale for the average number of carbons along the bottom, and also major compound peaks are labeled.
BROWSE the database, or SEARCH it. It's FREE!
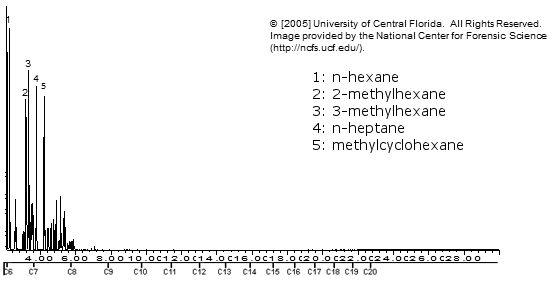
Ronsonol lighter fluid
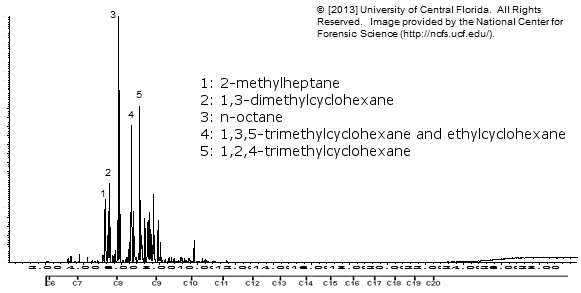
Zippo lighter fluid
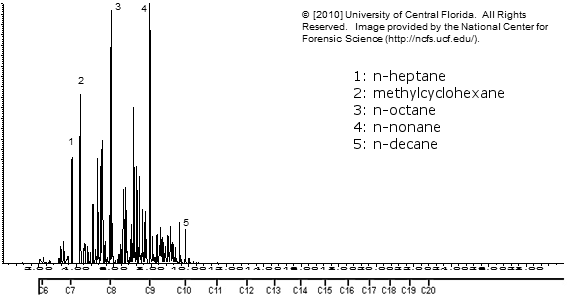
Klean Strip VM&P Naptha
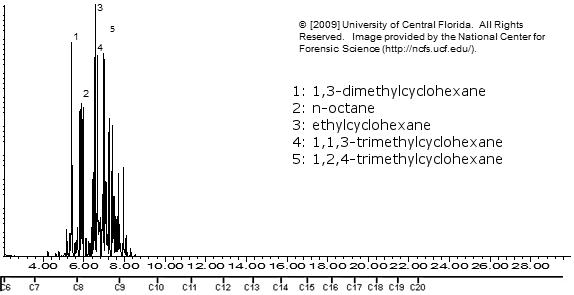
Note how similar the above 3 are!
Escort camp fuel
This is very light and volatile. It will evaporate very fast.
Exxon Sweet South Crude Oil
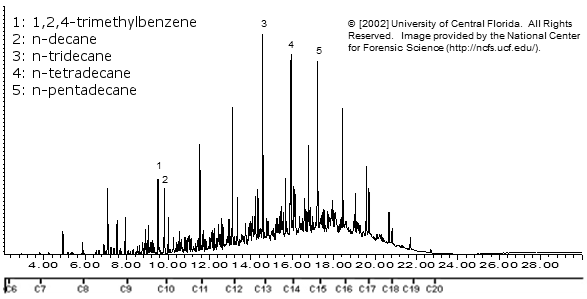
There are a lot of compounds here.
Mastercraft Varsol
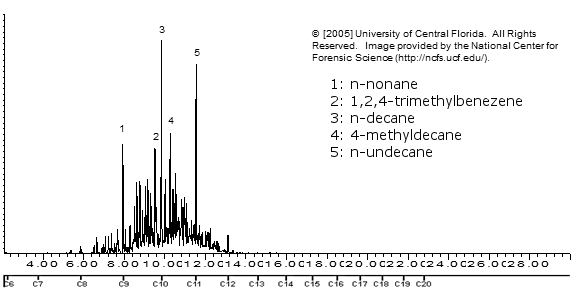
Note how varsol / stoddard solvent is mostly higher boiling (bigger) alkanes and also has some aromatics present. Those aromatics will dissolve DMT just like toluene/xylene, so freeze precipitation won't work.
Gasoline (93 octane, BP)
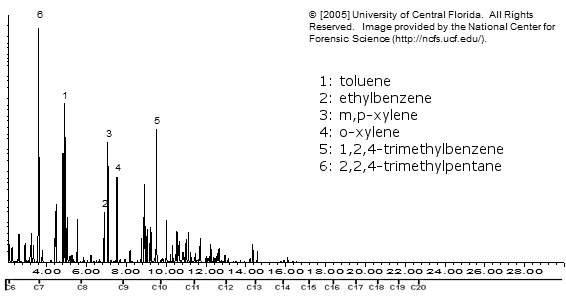
Just in case you were wondering what goes in a car engine.
& one more for lulz
WD-40
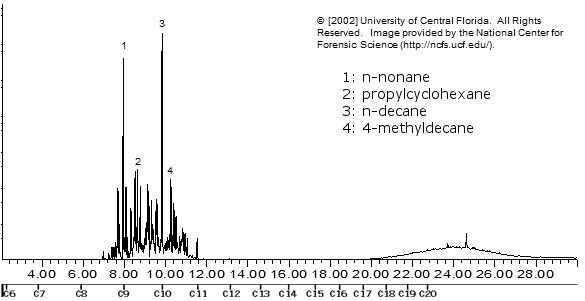
SECRETS REVEALED!!!! Actually, it's just petroleum distillate and some lubricating paraffin oil (the hump on the far right)
If you cannot read GC charts, here's a quick rundown: the GC is a machine that seperates compounds according to how volatile they are - you put a mixture into it and over time it will spit out the individual chemical compounds that make up that mixture. Usually, the same compound will always leave the GC at the same time - and it can be hooked up to a mas sspectrometer to get an exact identity.
GC charts are read like a bar graph: Lighter, smaller, more volatile compounds which exit the GC first appear on the left side. As the chart moves to the right, the compounds get progressively less volatile, heavier, and bigger. A bigger "peak" on the chart means more of the compound is present, and a smaller one means only a little was present (the amount of compound is proportional to the peak area, for you math nerds.) There is a bar scale for the average number of carbons along the bottom, and also major compound peaks are labeled.
BROWSE the database, or SEARCH it. It's FREE!
Ronsonol lighter fluid

Zippo lighter fluid

Klean Strip VM&P Naptha

Note how similar the above 3 are!
Escort camp fuel

This is very light and volatile. It will evaporate very fast.
Exxon Sweet South Crude Oil

There are a lot of compounds here.
Mastercraft Varsol

Note how varsol / stoddard solvent is mostly higher boiling (bigger) alkanes and also has some aromatics present. Those aromatics will dissolve DMT just like toluene/xylene, so freeze precipitation won't work.
Gasoline (93 octane, BP)

Just in case you were wondering what goes in a car engine.
& one more for lulz
WD-40

SECRETS REVEALED!!!! Actually, it's just petroleum distillate and some lubricating paraffin oil (the hump on the far right)
