LikeADaVinci
Bluelighter
- Joined
- Dec 31, 2023
- Messages
- 25
Hello bluelighters,
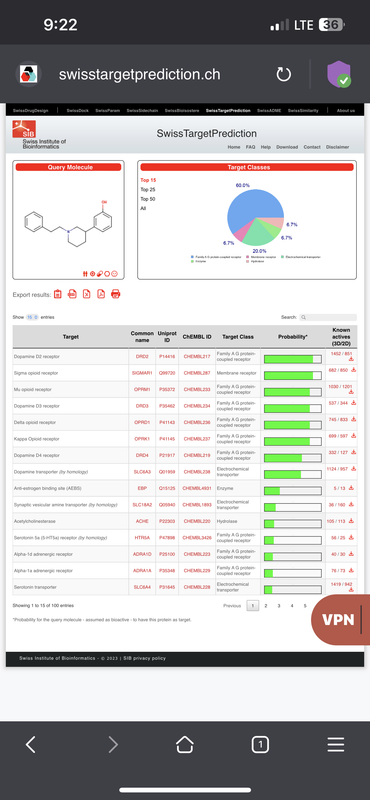
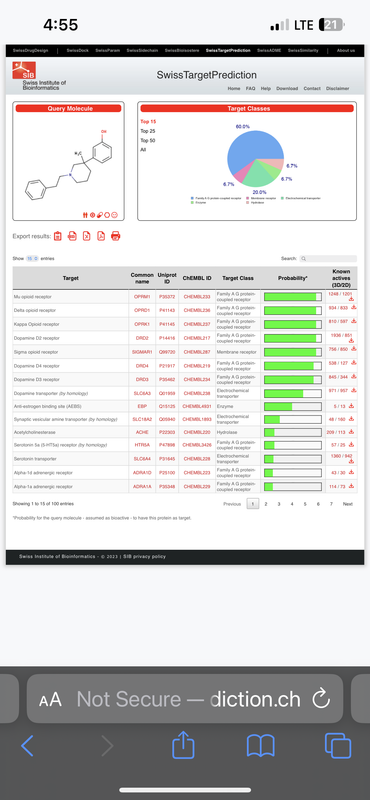
So, I’m curious as to Mu receptor agonists (pain-killing opioids) with strong dopaminergic activity, for the sake of developing an analgesic which blocks dopamine reuptake thereby stimulating the CNS and combating deadly respiratory depression inherit to opioids.
It also seems like the recreational potential of such a compound would be increased, since, you have the mu agonist activity, which inhibits GABA inter neurons in the ventral tagmental area thereby disinhibiting dopamine release in the accumbens, while simultaneously blocking the reuptake of dopamine in the accumbens.
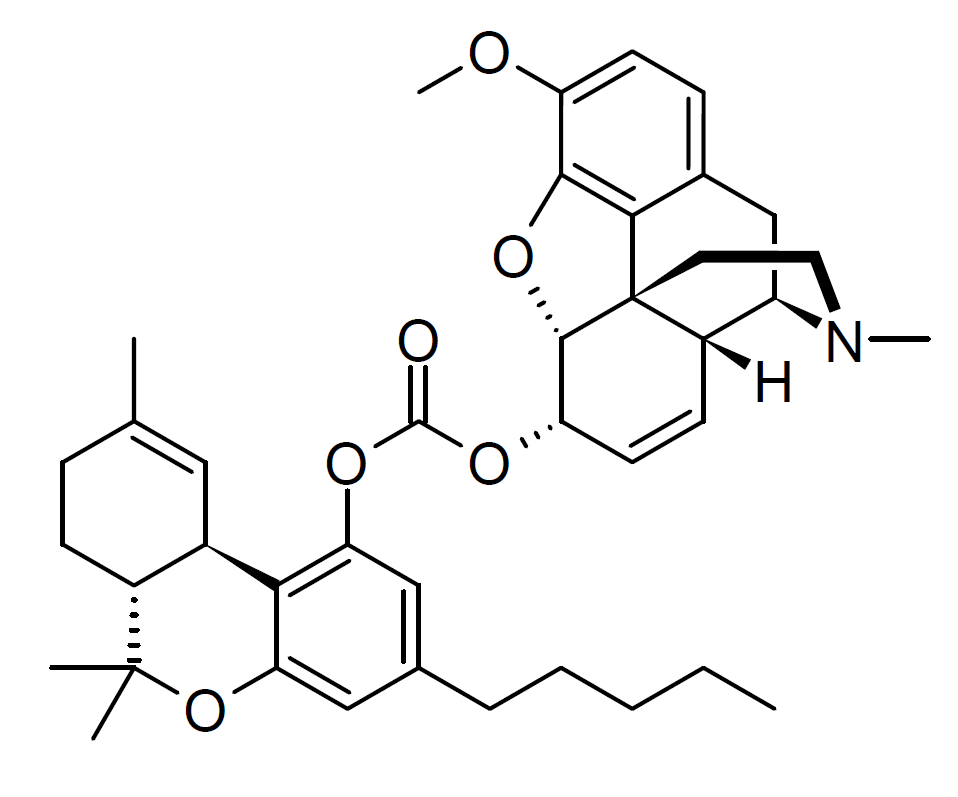
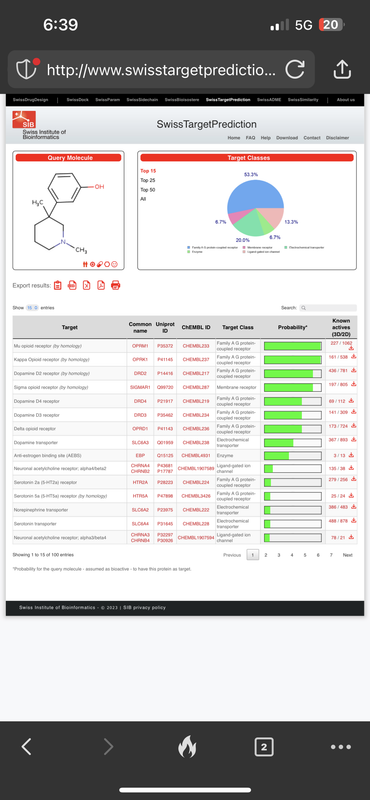
Take this compound for instance, and it’s activity profile:
 postimg.cc
postimg.cc
What do you think of such a combination of activities / receptor targets?
LikeADaVinci
So, I’m curious as to Mu receptor agonists (pain-killing opioids) with strong dopaminergic activity, for the sake of developing an analgesic which blocks dopamine reuptake thereby stimulating the CNS and combating deadly respiratory depression inherit to opioids.
It also seems like the recreational potential of such a compound would be increased, since, you have the mu agonist activity, which inhibits GABA inter neurons in the ventral tagmental area thereby disinhibiting dopamine release in the accumbens, while simultaneously blocking the reuptake of dopamine in the accumbens.
Take this compound for instance, and it’s activity profile:

IMG 2441 — Postimages
What do you think of such a combination of activities / receptor targets?
LikeADaVinci