Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
Coke analog shows "opposite function profile" to anti-addiction mechanism in ibogaine
3′-methoxy-8-methyl-spiro(8-azabicyclo(3.2.1)octane-3,5′(4′H)-isoxazole
(ibogaine reference in original source on "ledge" of image when it indents / cuts-in)
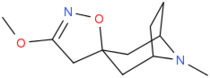
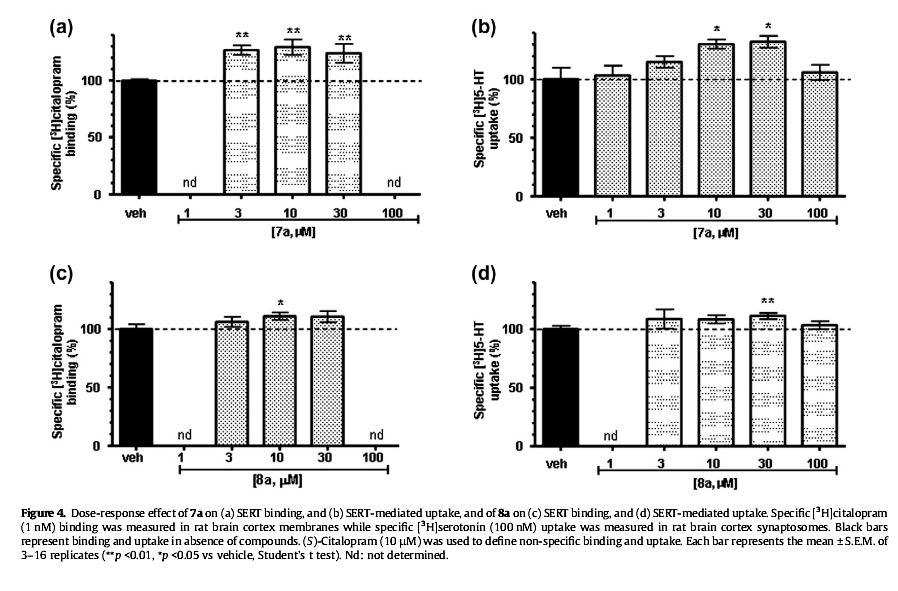
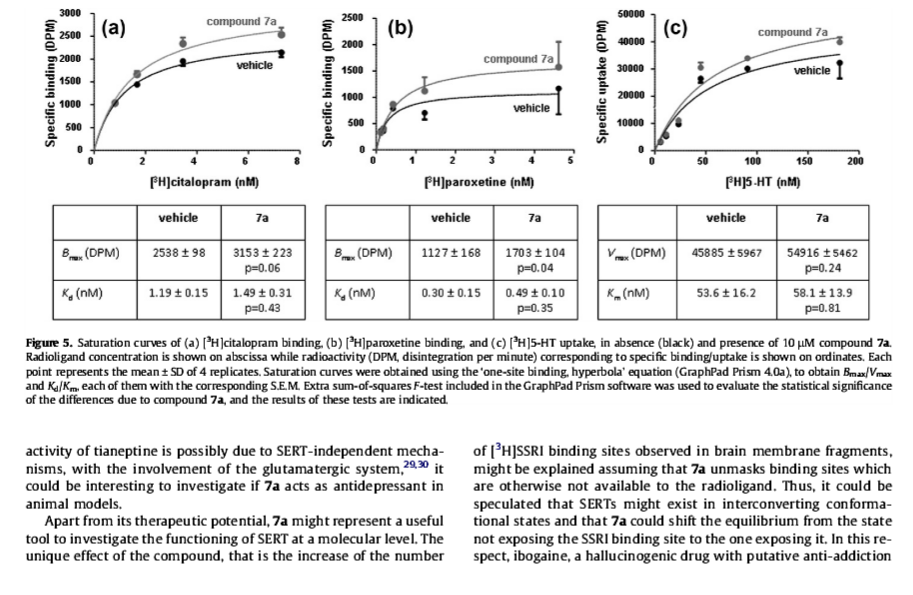
Reveals hidden transporters (because apparently, they aren't available at all times in suchwise for binding to occur by monoamine reuptake inhibitor ligands) by making them ready, 'priming their pump' (har-har; reuptake pump) so that they may be bound my a secondary reuptake inhibiting ligand readily and right away in full force by positioning the MAT in a configuration with which it may accept agonism/antagonism
Interestingly even very similar "spirocyclic tropanyl-Δ(2)-isoxazoline" cocaine-like analogue compounds do not do what this is shown to do, ultimately it is considered to have the opposite effect profile of the anti-addiction drug ibogaine, and displays in vitro (and therefore in a very objective, mechanistic manner in regard to MAT allosteric functionality) what tianeptine is the only other drug known to show to do and then only in vivo (so who knows what up/down-stream factors are at play)
Technically, it works by noncompetitive inhibition of 5-HT transporters decreasing Vmax with small change in the Km for serotonin in what results as stabilizing the cytoplasm-facing conformation of SERT, wherein that acts by interconverting the conformational state of unexposed SERTs to ones exposing the SSRI binding site via a shift to the equilibrium of the MAT by unveiling serotonin uptake-area ligand-sites on surface of transporter that allows for binding by (adjunct) exogenous ligand, when SERT is otherwise conformed in a transitional manner where a SERT ligand cannot bind, this effect with compound in question occurs at concentrations of 10μM—30μM creating the allosteric effect of *making more instances of MAT available for binding* (while exerting an inhibitory orthosteric effect when concentrations reach >30μM and above.)
No other known drug does this in this way.... and, it's a cocaine analog... with the diametrically opposed putative method of action to ibogaine's anti-addictive properties.... Can you say, most reinforcing / addicting drug ever? ;-P (or perhaps, one must be found to focus this effect on DAT rather than SERT, then we'd see. =-P )


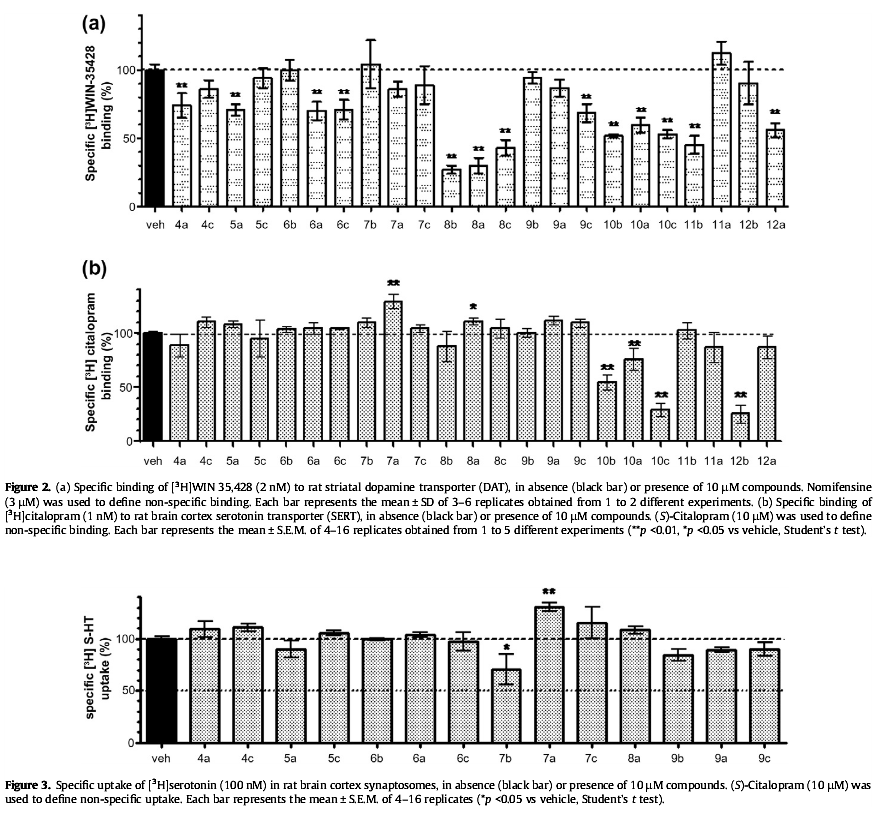
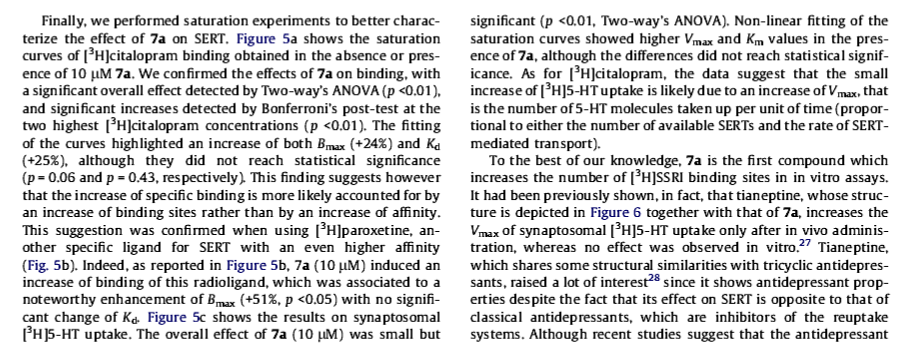

3′-methoxy-8-methyl-spiro(8-azabicyclo(3.2.1)octane-3,5′(4′H)-isoxazole
(ibogaine reference in original source on "ledge" of image when it indents / cuts-in)

Reveals hidden transporters (because apparently, they aren't available at all times in suchwise for binding to occur by monoamine reuptake inhibitor ligands) by making them ready, 'priming their pump' (har-har; reuptake pump) so that they may be bound my a secondary reuptake inhibiting ligand readily and right away in full force by positioning the MAT in a configuration with which it may accept agonism/antagonism
Interestingly even very similar "spirocyclic tropanyl-Δ(2)-isoxazoline" cocaine-like analogue compounds do not do what this is shown to do, ultimately it is considered to have the opposite effect profile of the anti-addiction drug ibogaine, and displays in vitro (and therefore in a very objective, mechanistic manner in regard to MAT allosteric functionality) what tianeptine is the only other drug known to show to do and then only in vivo (so who knows what up/down-stream factors are at play)
Technically, it works by noncompetitive inhibition of 5-HT transporters decreasing Vmax with small change in the Km for serotonin in what results as stabilizing the cytoplasm-facing conformation of SERT, wherein that acts by interconverting the conformational state of unexposed SERTs to ones exposing the SSRI binding site via a shift to the equilibrium of the MAT by unveiling serotonin uptake-area ligand-sites on surface of transporter that allows for binding by (adjunct) exogenous ligand, when SERT is otherwise conformed in a transitional manner where a SERT ligand cannot bind, this effect with compound in question occurs at concentrations of 10μM—30μM creating the allosteric effect of *making more instances of MAT available for binding* (while exerting an inhibitory orthosteric effect when concentrations reach >30μM and above.)
No other known drug does this in this way.... and, it's a cocaine analog... with the diametrically opposed putative method of action to ibogaine's anti-addictive properties.... Can you say, most reinforcing / addicting drug ever? ;-P (or perhaps, one must be found to focus this effect on DAT rather than SERT, then we'd see. =-P )








Last edited:

 ;-P and without an instructor beaming with "I taught that fellow", hell to the academic establishment. ;-P I have a theory on electron energy resonance and action potential alterations and energy exchange in vivo for ligands with both affinity for high-through-put charged channels, and voltage gated receptor affinity of some kind; obviously coke inspired. ;-j )
;-P and without an instructor beaming with "I taught that fellow", hell to the academic establishment. ;-P I have a theory on electron energy resonance and action potential alterations and energy exchange in vivo for ligands with both affinity for high-through-put charged channels, and voltage gated receptor affinity of some kind; obviously coke inspired. ;-j )