Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
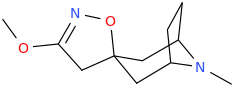
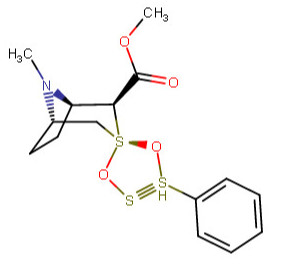
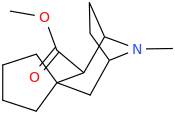
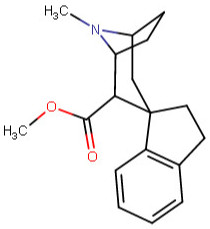
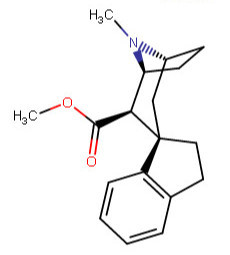
These spirocyclic tropanyl-Δ(2)-isoxazolines reportedly enhance re-uptake inhibitor ligands at SERT & DAT. Does anyone have access to a source that could give me the IUPAC to as many of these and if possible draw them up in molecular-2D for me? I'd like to contribute them to WP.