Fertile
Bluelighter
- Joined
- Mar 31, 2022
- Messages
- 1,627
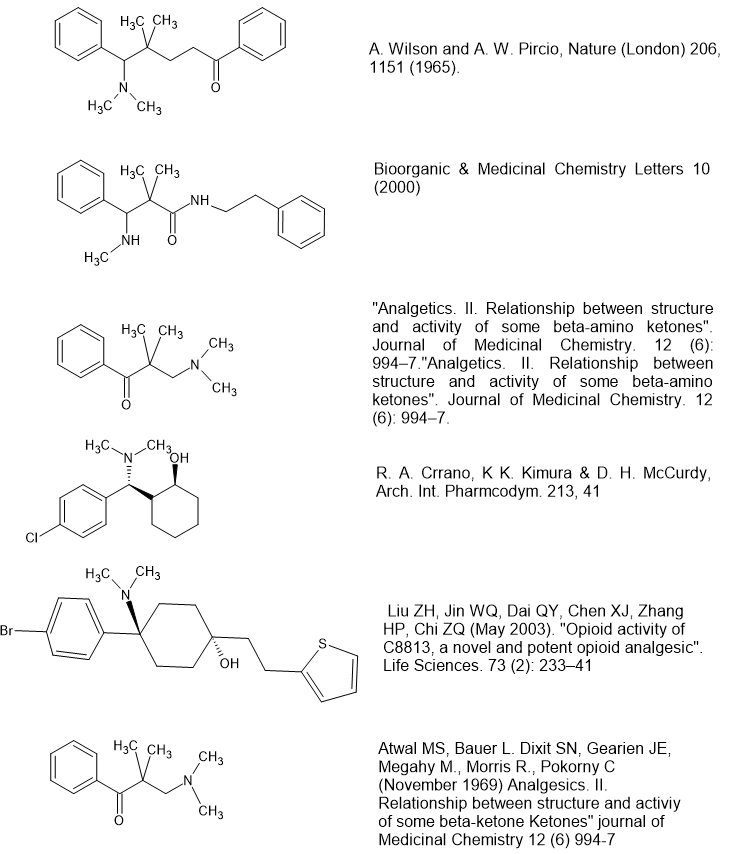
Although it's far from accurate, older literature often quotes 'the morphine rule' which is:
-Aromatic system
-Quaternary Carbon
-2 carbon chain
-Tertiary amine
They even added 'the fentanyl modification' to get around the unpalatable fact that whole new classes were turning up that didn't follow 'the rules'. But I've also noted examples in which the lone-pairs and the N: lone-pair are swapped spacially. It's interesting and MANY classes of opioid follow this alternative set of rules. People wondering about the ciramadol analogue in which the meta phenol is swapped for a para -Cl should note that I found it in Reaxys. I couldn't found the reference other than to say that it's as potent as ciramadol BUT it's a full agonist.
lone-pairs and the N: lone-pair are swapped spacially. It's interesting and MANY classes of opioid follow this alternative set of rules. People wondering about the ciramadol analogue in which the meta phenol is swapped for a para -Cl should note that I found it in Reaxys. I couldn't found the reference other than to say that it's as potent as ciramadol BUT it's a full agonist.
Now a question for you all - note that BDPC requires that p-Br (or a p--Me which I BET is going to be much more euphoric due to altered pharmokinetics). But it's interesting that the difference in atomic radius of -Br & -CH3 are not the same.... a -CF3 would be MUCH closer. Also, if you swap the p-Br (or p-CH3) for a m-OH, you end up with an antagonist (work from Dan Lednicer) and so I suspect that the propanamide derivatives, the only potent ones having a m-OH so are antagonists will likewise become full agonists if a p-Br/p-Cl/p-CH3/p-CF3 or similar (you would need to chectk them all because no calculation I know of can help decide the most active.
For those who haven't read it, it's IMPOSSIBLE to confer antagonist properties on an opioid that does not possess a phenol or bioisostere. What bioisostere? Well a carboxamide is the best known example. Swap the 3-OH of morphine for a -CONH2 and it's only 1/2 the potency but the duration is 12 hours which I sense might be of use in the design of opioids.
I would like to thank Skorpio for all of the papers he has obtained for me - if this was a publication, I certainly wouldn't refer to him as 'et al'. Also thankyou for Fast & Bulbous for correcting my mistakes and having no end of tolerance towards my stupidity.
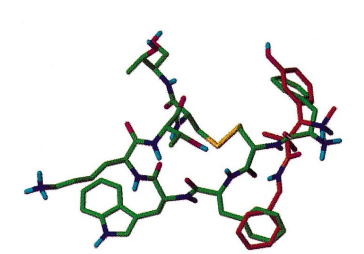
Also, for what it's worth, the phenylpropanamide opioid overlaying the MOR. Now WHY should a para substitution alter affinity? Well, the days of lock-and-key have gone, now science believes that a drug allows the receptor to adopt a lower energy state and that the active conformation of a drug might be quite unexpected.
I'm too old - my education is from the 80s & 90s so I'm hoping younger BL chemists can chip in and provide further insights. So many unanswered questions.
-Aromatic system
-Quaternary Carbon
-2 carbon chain
-Tertiary amine
They even added 'the fentanyl modification' to get around the unpalatable fact that whole new classes were turning up that didn't follow 'the rules'. But I've also noted examples in which the
Now a question for you all - note that BDPC requires that p-Br (or a p--Me which I BET is going to be much more euphoric due to altered pharmokinetics). But it's interesting that the difference in atomic radius of -Br & -CH3 are not the same.... a -CF3 would be MUCH closer. Also, if you swap the p-Br (or p-CH3) for a m-OH, you end up with an antagonist (work from Dan Lednicer) and so I suspect that the propanamide derivatives, the only potent ones having a m-OH so are antagonists will likewise become full agonists if a p-Br/p-Cl/p-CH3/p-CF3 or similar (you would need to chectk them all because no calculation I know of can help decide the most active.
For those who haven't read it, it's IMPOSSIBLE to confer antagonist properties on an opioid that does not possess a phenol or bioisostere. What bioisostere? Well a carboxamide is the best known example. Swap the 3-OH of morphine for a -CONH2 and it's only 1/2 the potency but the duration is 12 hours which I sense might be of use in the design of opioids.
I would like to thank Skorpio for all of the papers he has obtained for me - if this was a publication, I certainly wouldn't refer to him as 'et al'. Also thankyou for Fast & Bulbous for correcting my mistakes and having no end of tolerance towards my stupidity.

Also, for what it's worth, the phenylpropanamide opioid overlaying the MOR. Now WHY should a para substitution alter affinity? Well, the days of lock-and-key have gone, now science believes that a drug allows the receptor to adopt a lower energy state and that the active conformation of a drug might be quite unexpected.

I'm too old - my education is from the 80s & 90s so I'm hoping younger BL chemists can chip in and provide further insights. So many unanswered questions.



