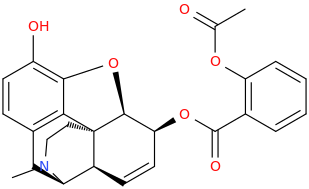
Wouldn't the diester be tricky to form, due to steric hindrance?
And believe you me, I don't hate dipropionylmorphine. Its one of my favourite of all opioids, only opium isolate, the propionyl ester of the bulk of phenolic material present in such an isolate, prepped for IV, minus codeine, I'm a big fan. I just don't like the stink of the halide. I can live with it. I mean really, its of little consequence. Its just unpleasant. I spent most of today up to my ass in ammonia, diethyl ether and more fucking ammonia. Worst of all though was accidentally sucking naphtha fumes through the tip of an E-cig accidentally wetted with a little of same. Disgusting. Well I say naptha, white spirit, just used as an inert to keep something behaving itself temporarily. Stinks though. h
As far as potency, etc goes, dipropionylmorphine beats the SHITE out of diamorphine, whilst picking up a bit of that histamine release on injection lacking in diamorphine, and its both potent, packs one hell of a rush on IV, and DAMN does it last a long time. Be lucky to get 6-7 hours of pain relief, withdrawal delay and mobility out of IV morphine. 12 easily with dipropionylmorphine, IV. Aside from opium and acylated (propionyl) 'kompot', only refined with the benefit of decent tech. The ONLY opioid thats ever hit the spot, has been methadone. And not talking cut street skag, Tried it, could give less of a shit about it. But something that can be vouched for and known not to be cut. H just doesn't. It falls short of expectactions. I'd be most curious to know if ever DPM has been assayed in tandem with morphine and H at MOR splice variants and heterodimers/higher order oligomeric receptors if they exist for MORs. Because there are notable differences in the specificity of H for certain exons present in alternately spliced MOR isoforms compared to morphine.

 extra analgesia ftw
extra analgesia ftw