It is not that. The OP, I.e me, was complaining that the person who is advertising this substituted P2P for sale, did not label it correctly, and the OP, had to enquire as to the precise nature of the compound. In my defence, it should be noted that I had been working in my lab for 3-4 days non-stop, with no more rest than the occasional cold glass of iced lime juice, the occasional rollup and brief pause in activity to smoke the same. Non-stop, the occasional toilet break yes, but not a nanosecond of sleep. And his last opiate dose was wearing off when he posted that, tired and exhausted.
Been struggling with a particularly intractable workup, that IMO will need steam distillation or total evaporation of solvent and bulk water. Will need a place to set up the vacuum pump, and he couldn't do that in the dead of night for it would have woken his father with the noise of its operation, and he has done nothing to deserve my breaking into HIS rest. He is not a chemist and did not choose to be awoken by the noise of MY chemistry endeavours.
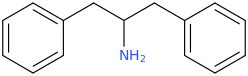
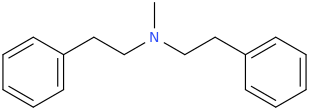
IUPAC is not my problem. It was the issue of the seller not labelling his compound clearly.
So, what are known about 'aphphetamine', or the N-methyl, N-ethyl, N-cyclopropyl or N-cyclopropylmethyl and N-various pentyl derivatives and any of the equivalent cathinones or phentermines? info on ANY of these will be appreciated. The pyrovalerone analog especially interests. As do the N-methyl, N-ethyl and N-unsubstituted primary amine cathinones protected as the pthalimidopropiophenones.