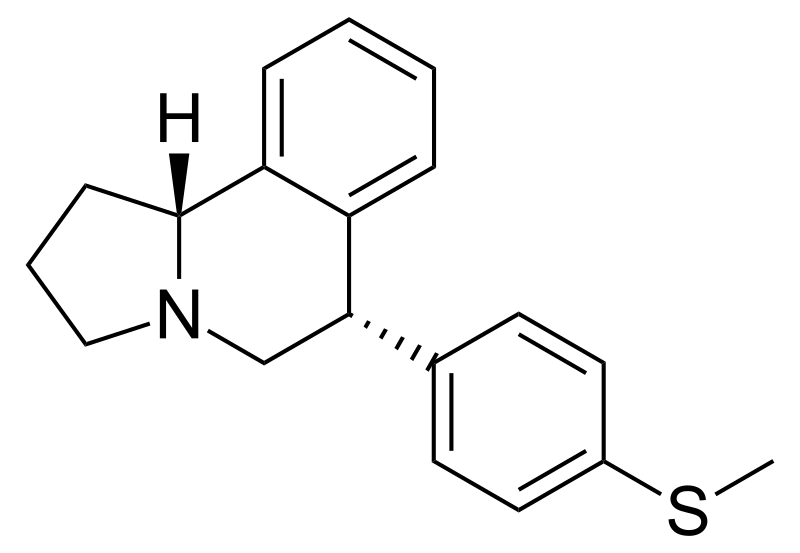
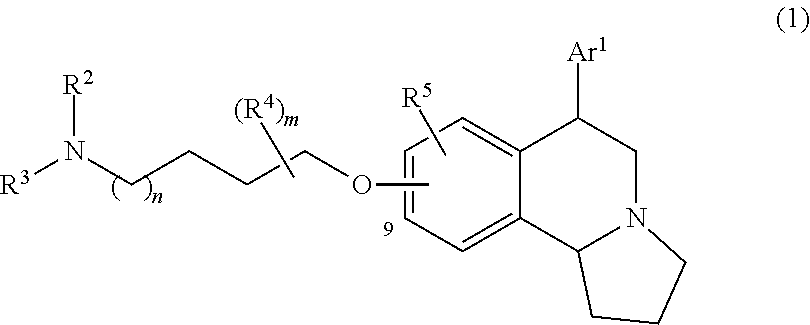
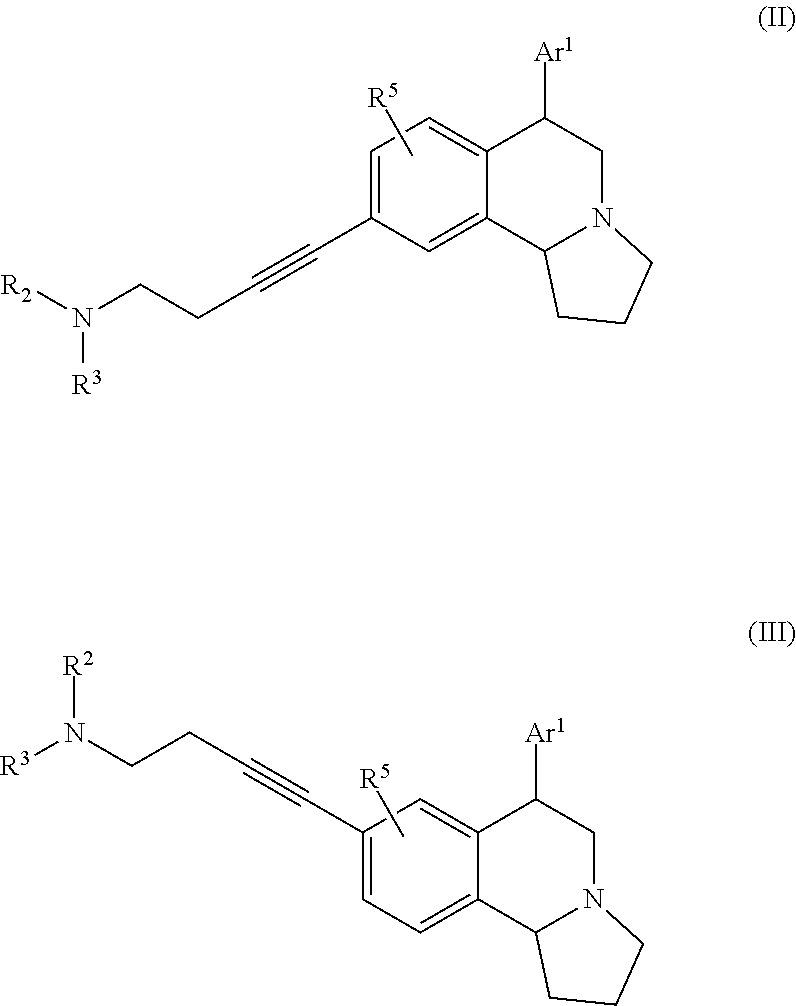
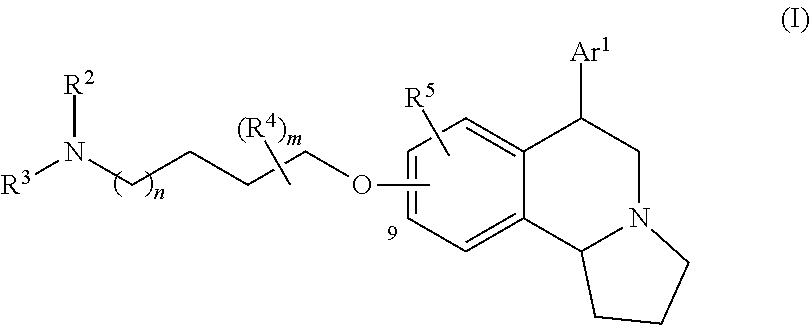
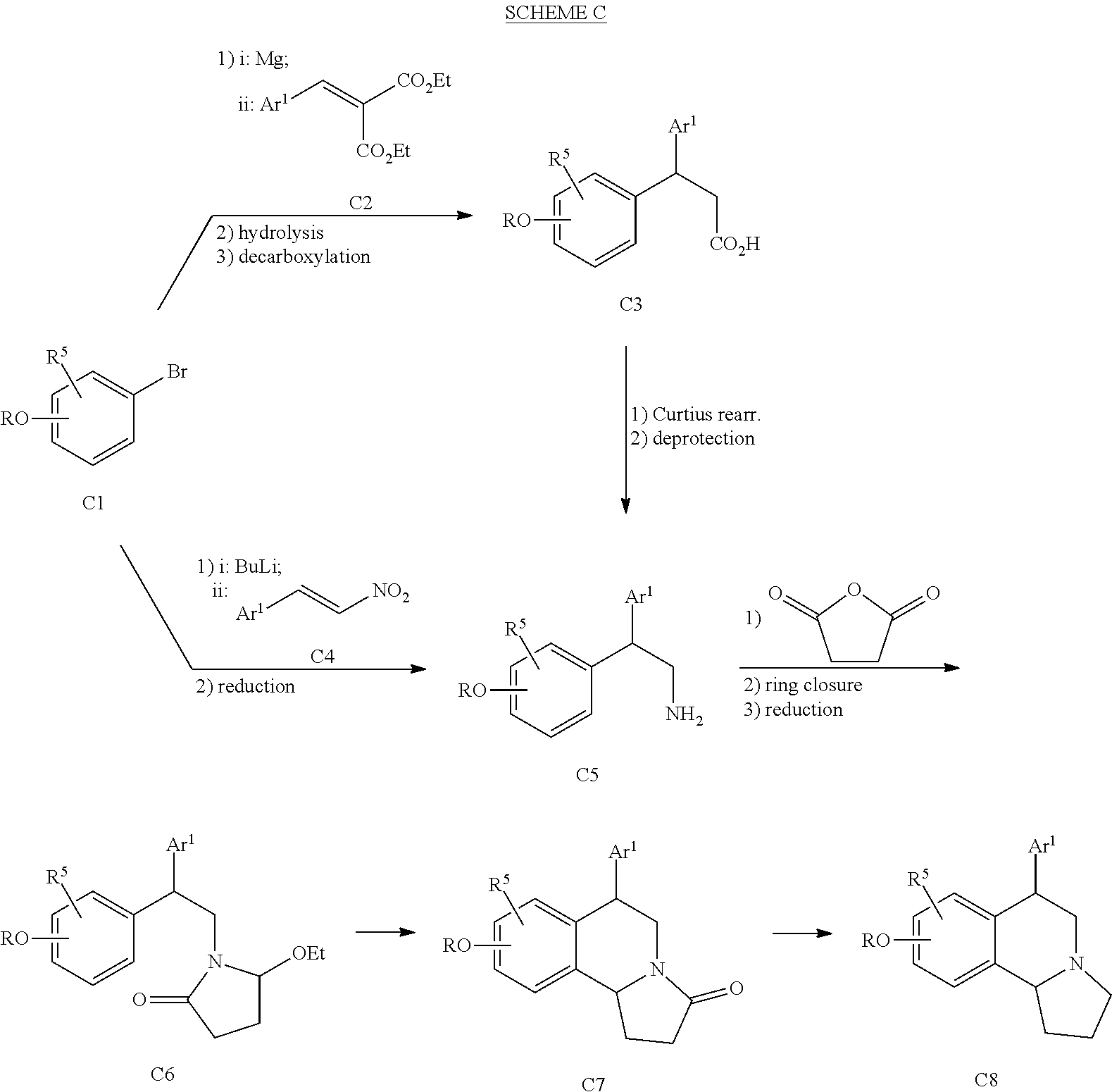
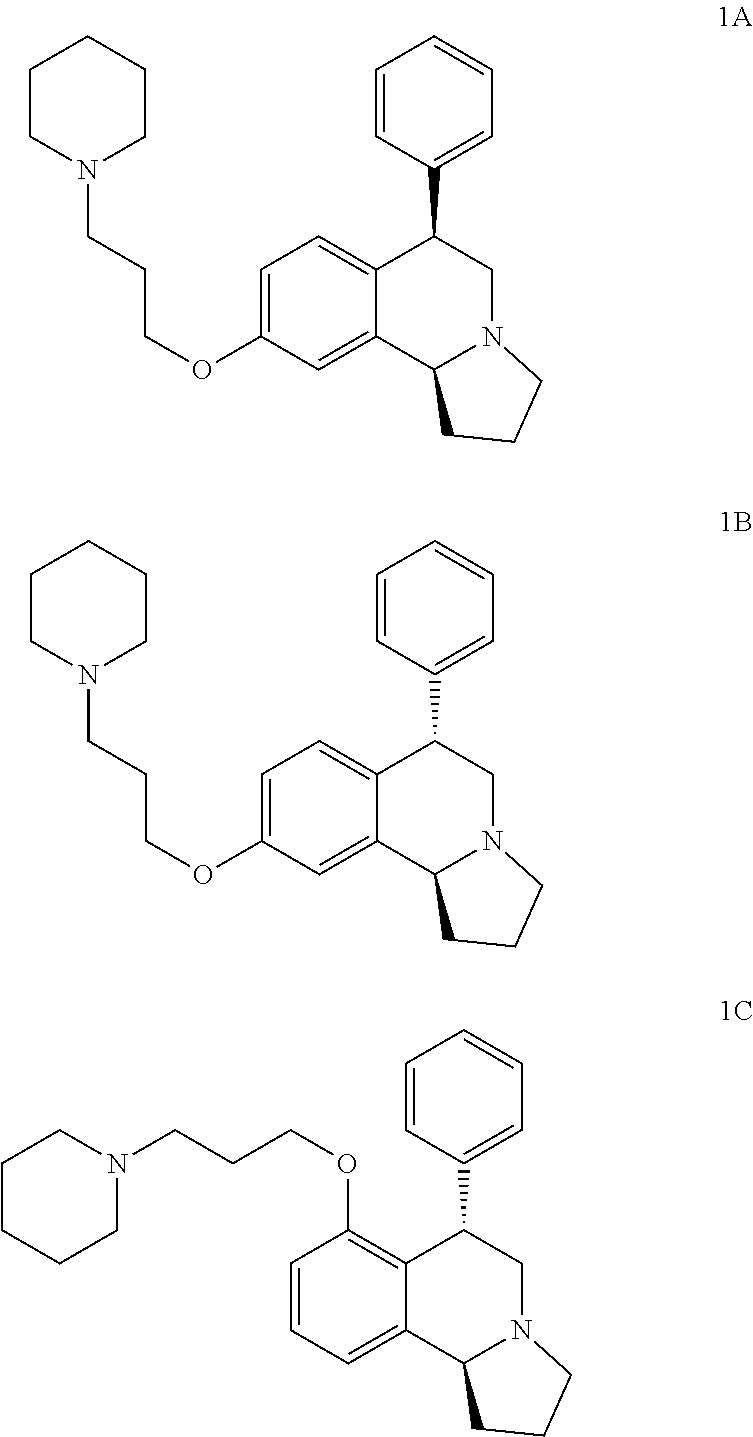
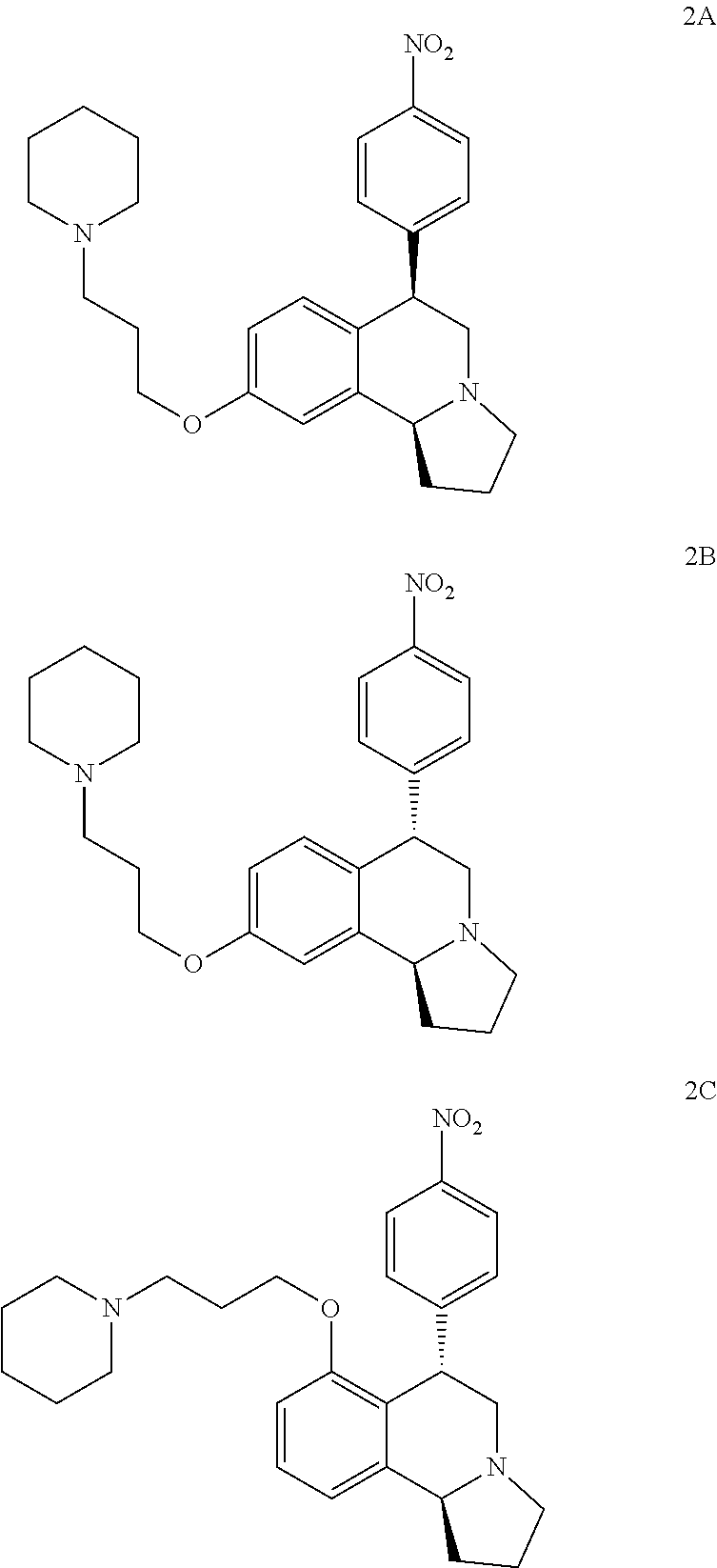
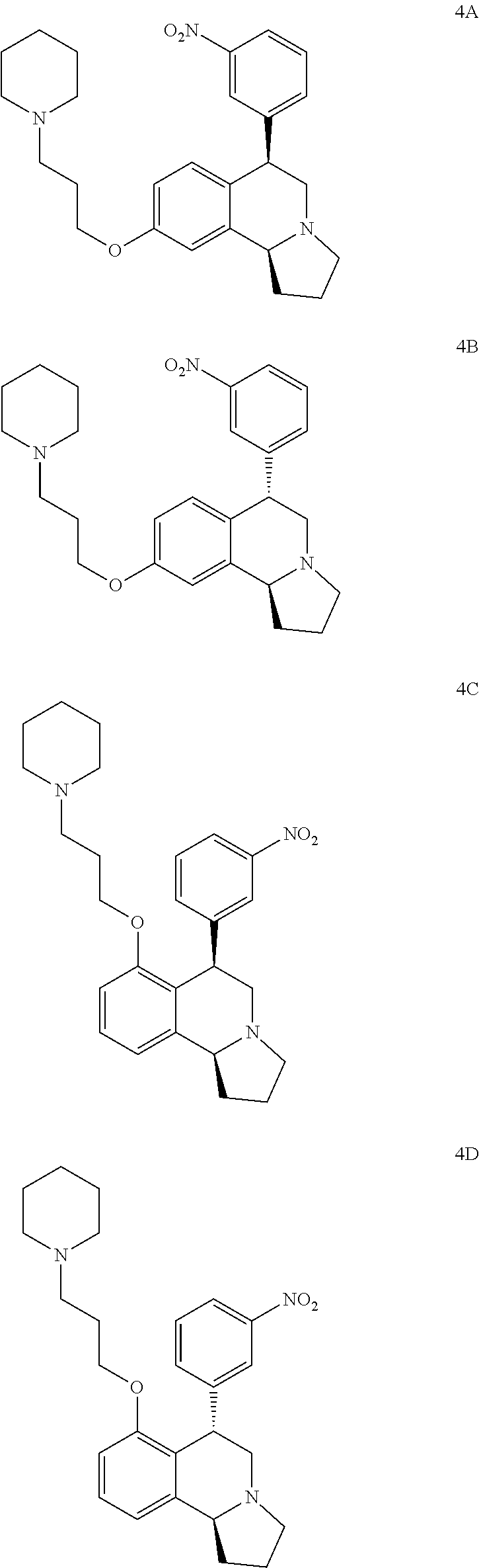
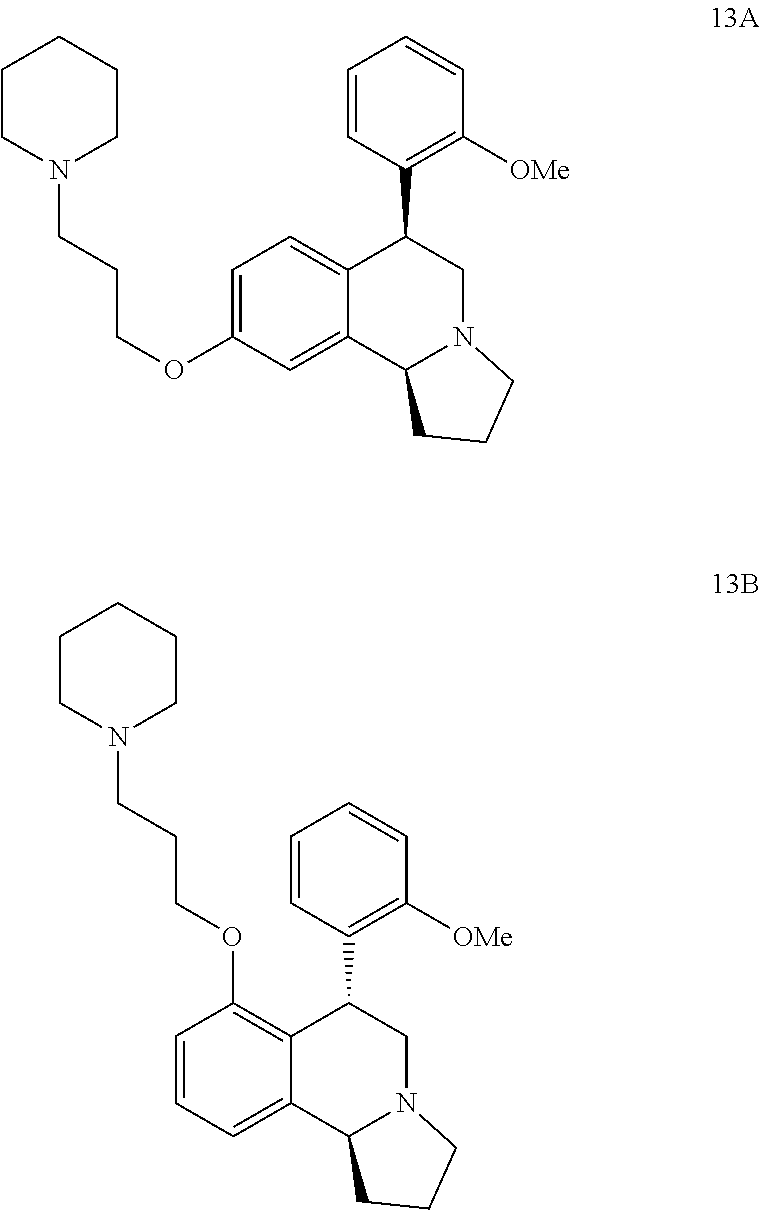
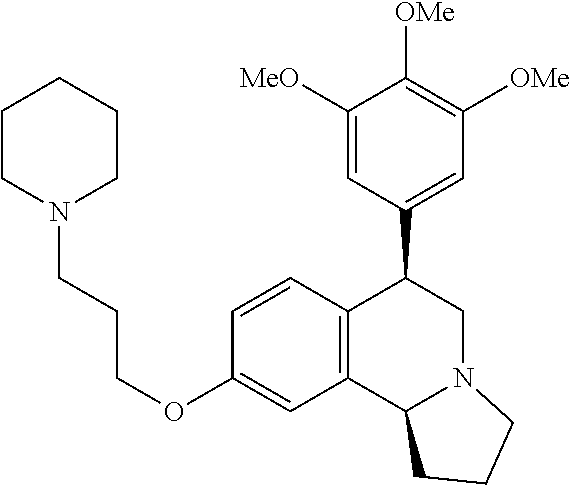
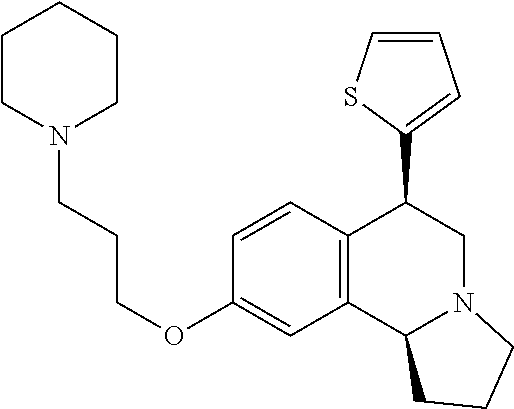
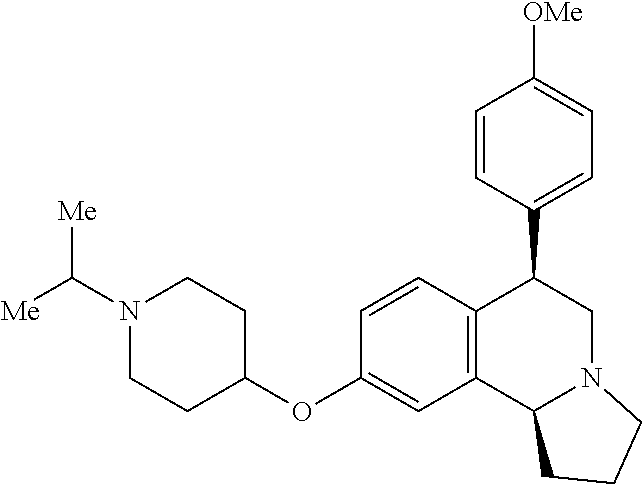
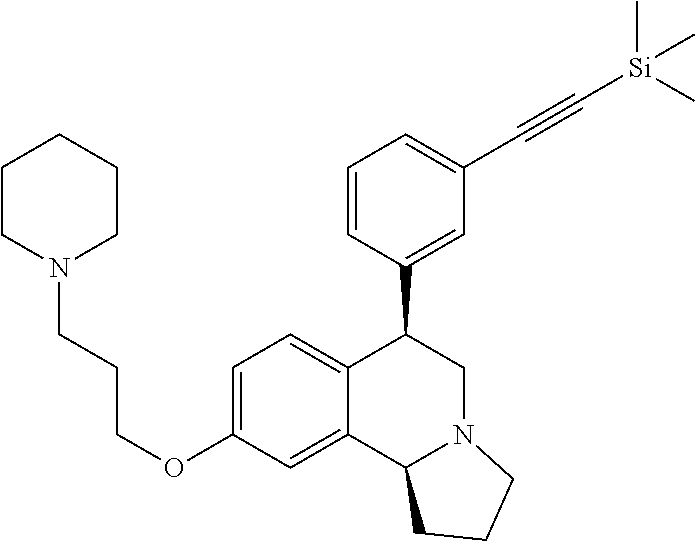
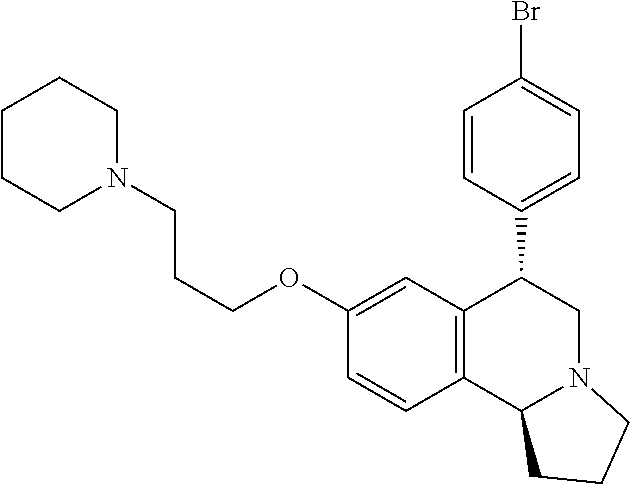
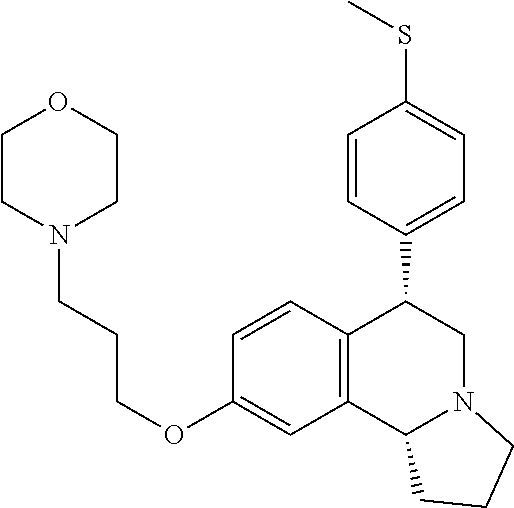
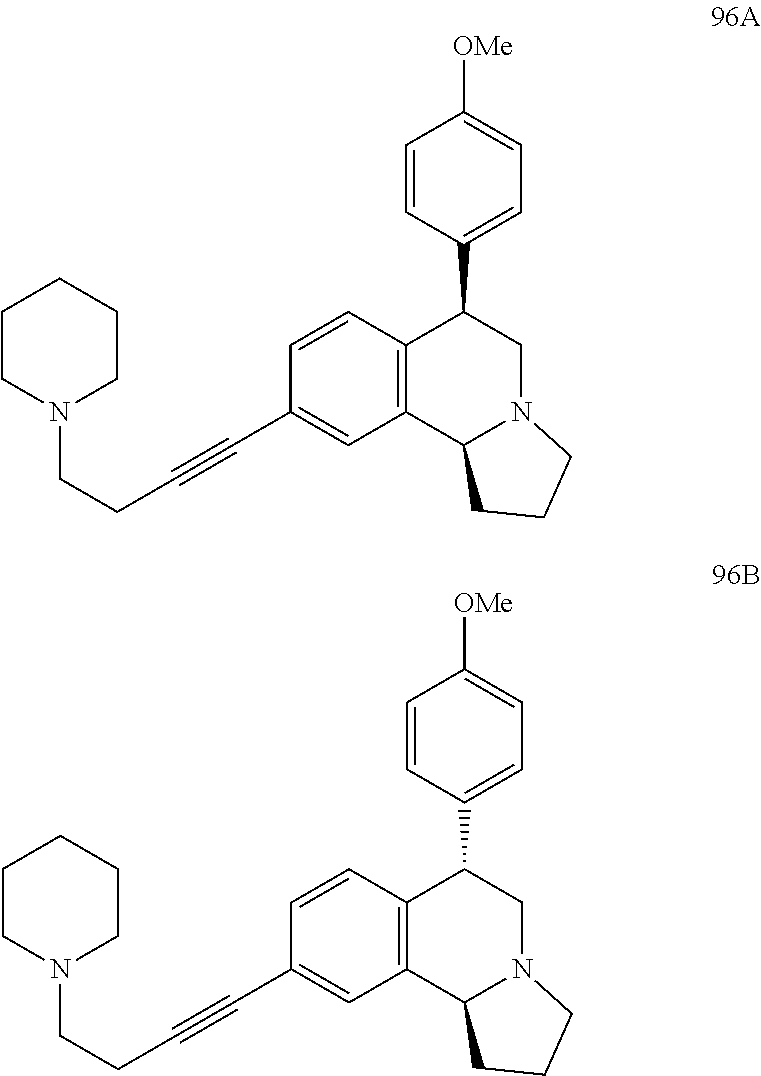
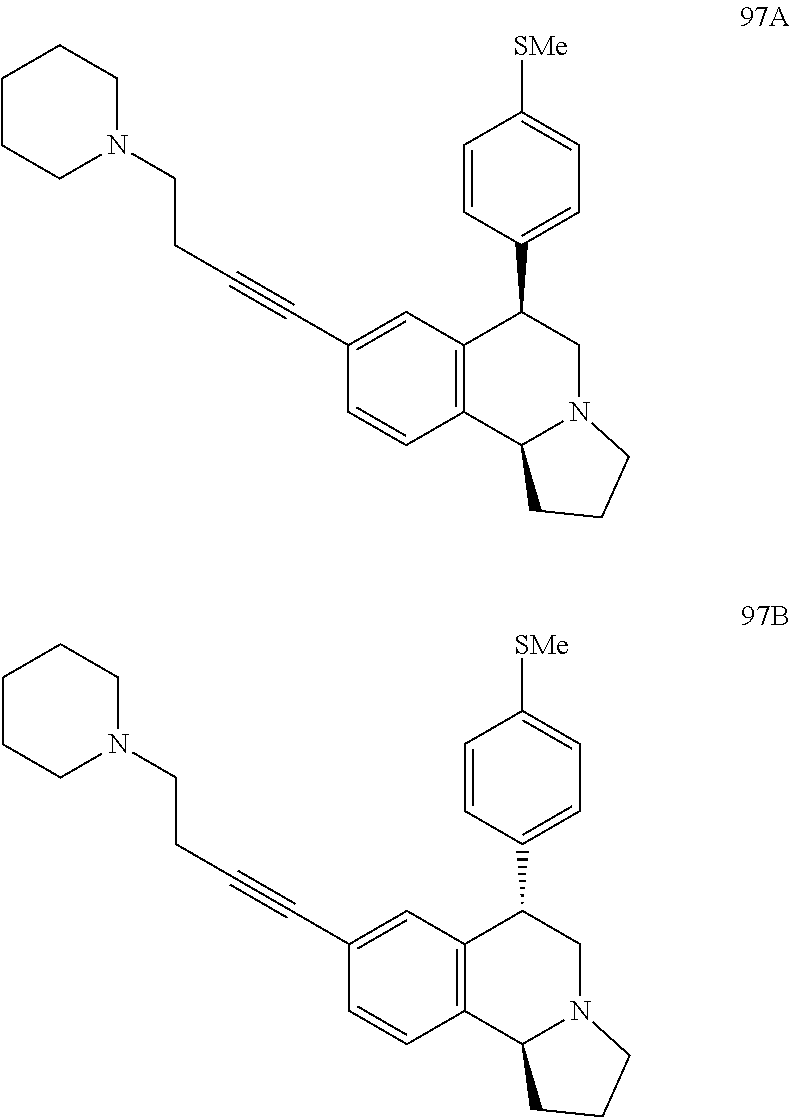
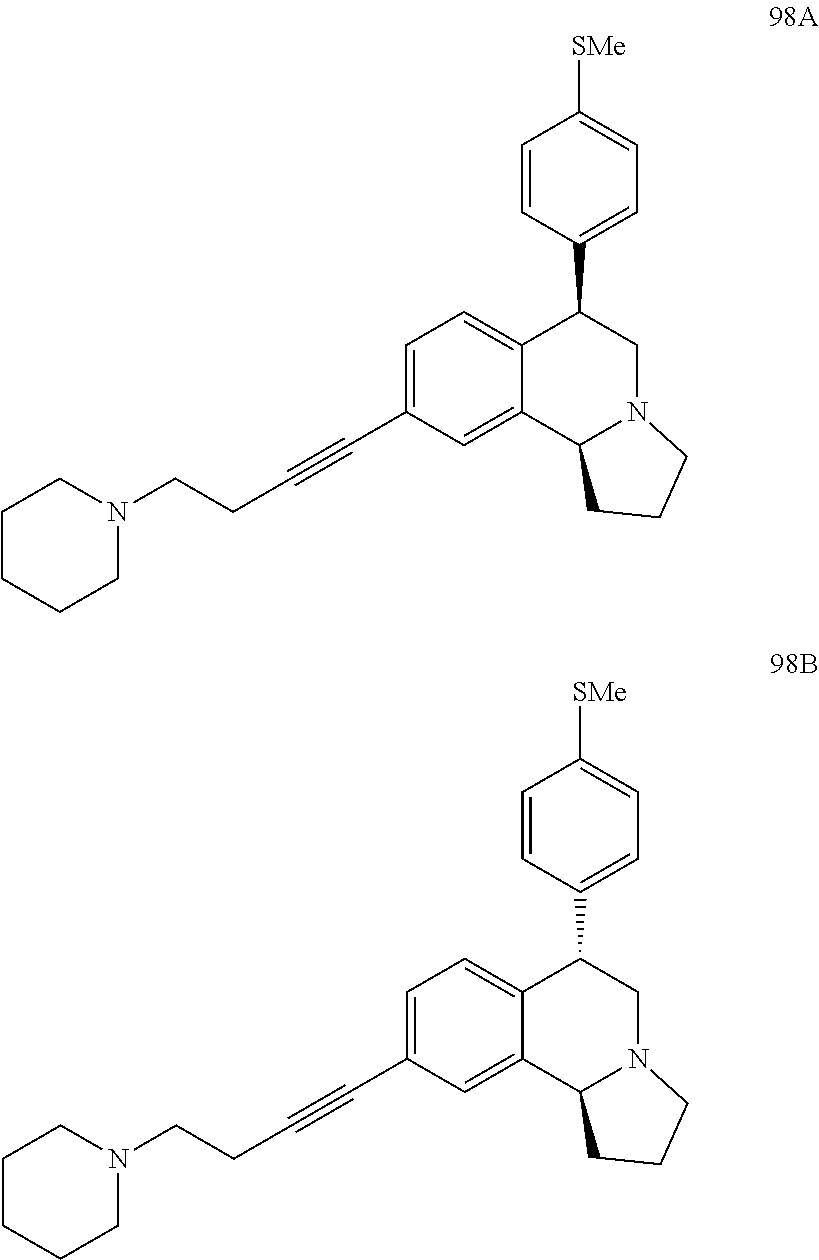
US 4908450 'Certain hexahydro-6-arylpyrrolo[2,1-A]isoquinolines'
US 4595688 'Hexahydropyrrolo[2,1-a]isoquinoline derivatives and antidepressant use thereof'
People will notice that the above are a development of:
US Patent 3947456 'Substituted 4-Phenyl Isoquinolines'
Themselves developed from and related i.e. poperidine ring swapped for morpholine ring or thiomorpholine ring.
US Patent 2820038 - 2-Diphenyl-Methyl-Piperidine
Which are simple ring-expansions of.
Taking us all the way back to:
AMPHETAMINES
US 4595688 'Hexahydropyrrolo[2,1-a]isoquinoline derivatives and antidepressant use thereof'
People will notice that the above are a development of:
US Patent 3947456 'Substituted 4-Phenyl Isoquinolines'
Themselves developed from and related i.e. poperidine ring swapped for morpholine ring or thiomorpholine ring.
US Patent 2820038 - 2-Diphenyl-Methyl-Piperidine
Which are simple ring-expansions of.
Taking us all the way back to:
AMPHETAMINES