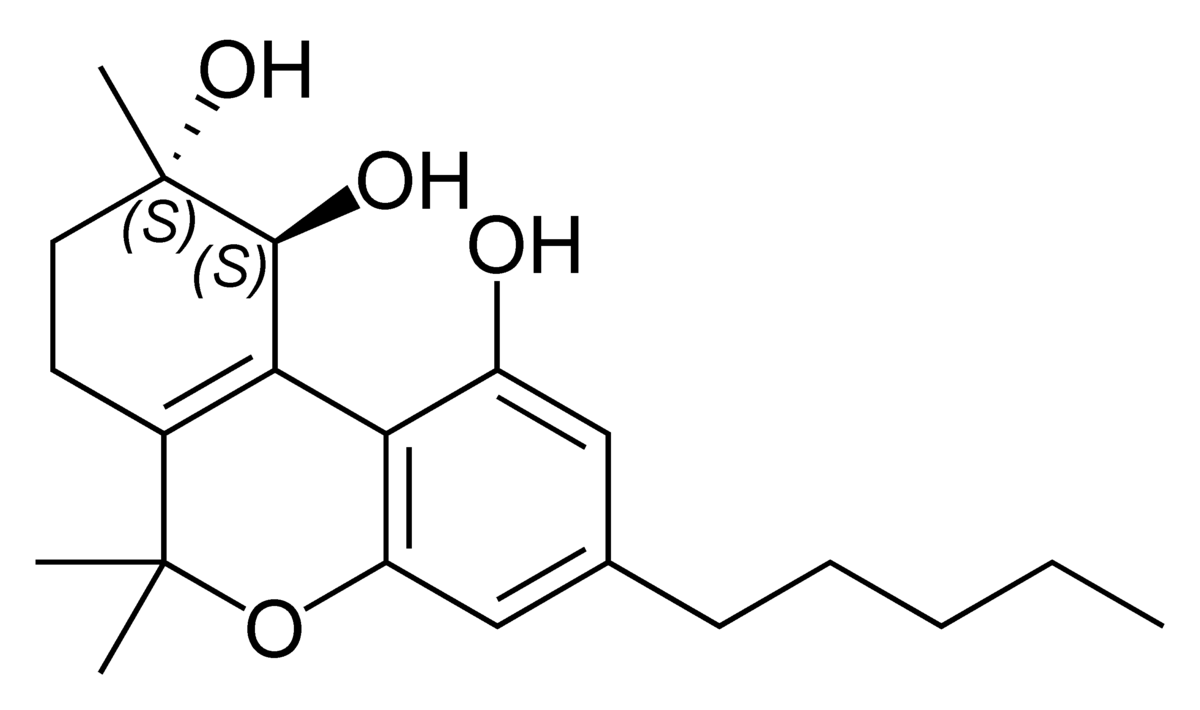
Well delta 8 THC is found naturally in the plant admittedly at much lower doses. When you compare them, a double bond moves ta slightly lower energy state and KEY is that all the patents do is to heat delta 9 to get delta 8 so one would presume that if you heat delta 9 (like smoking or vaping it), some of it will will convert into delta 8.
A solvent-free method for converting CBD or delta-9 THC-A to delta-9 THC and delta-8 THC includes adding CBD to a reaction vessel, streaming an inert gas through the reaction vessel, heating the CBD while stirring to melt the CBD, stirring the melting CBD, adding concentrated hydrochloric acid...
patents.google.com
Now, HOW people carry out the conversion is important as unwelcome compounds may be formed or traces of reagent and/or solvent might be left in there. My point is that delta 8 isn't toxic BUT something toxic is creeping in there.
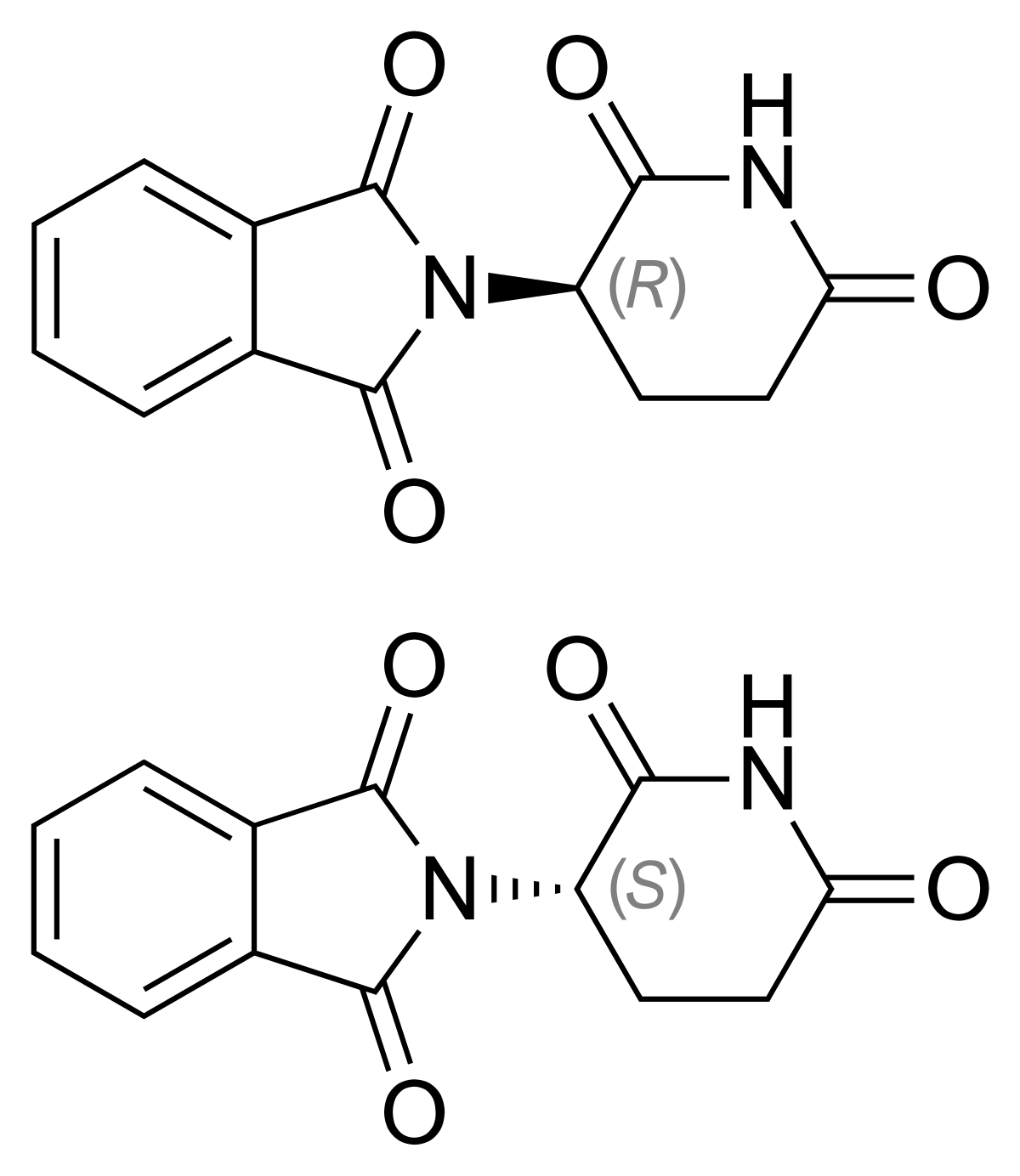
I know that one route was to dibrominate & then derominate to add a doule-bond and if some of the brominated intermediate made it's way to the end of the synthesis, it could be dangerous.
Are we sure that racemization doesn't occur when some methods are used.
There have been studies into delta 8 usage and no negative reports emerged.
Delta-8 THC products are not approved by the FDA and may put you at risk.

www.fda.gov
All the 'toxic symptoms' sound exactly like someone taking too much. Panic attacks. The wording aout the single child seems odd.
'One pediatric case was coded with a medical outcome of
death.'
Well, Google how many kids died after eating gummi bears laced with delta 9.....
Now, if that was unintentional and the child has asthma, a heart defect or other condition that would contraindicate using even delta 9 THC, I can well see the psychological effects resulting in dangerous changes to the body.
FYI I have tried delta 8 brownies as UK law isn't clear on a natural product being controlled by the PSA. It was very mild BUT the maker was an expert and we just got a light buzz, nothing major. Delta 8 is slightly LESS potent but maybe dose-response curve is steeper?
There are no comparisons with hospital admittions due to delta 9 THC so we have no way of gauging if overdoses/panic attacks are more common with delta 8.
Bottom line - the FDA is trying to provide a reason to ban delta 8 so that states that didn't make cannabis legal aren't discovering that someone found a way around the law. Delta 8 most certainly HAS been tested. The conclusion was that it was no more toxic than delta 9.
It's politics, not facts.
That is my opinion.
As for thalidomide (or aminorex or rofecoxib or permoline), history is littered by drugs whose danger could only be detected after years or even decades and I cannot help thinking that by now, if delta 8 really was toxic, it would have been spotted long ago.
I'm quite willing to be wrong but tobbaco and ethanol are VERY toxic in comparison and I don't see such shrill articles from roadsheets who should know better.
Personally, I wouldn't worry about it if I obtained the cookies from a reliable Dutch source. Since the Dutch make it illegal to sell delta 9 to tourists, they have also been selling delta 8.... I don't see any Dutch scare stories...
If you aren't sure, send it to me and I assure you it will be WELL tested!!!