-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
I Like to Draw Pictures of Random Molecules
- Thread starter nuke
- Start date
- Status
- Not open for further replies.
Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
Drug Design 101:
Chapter 1: STIMS
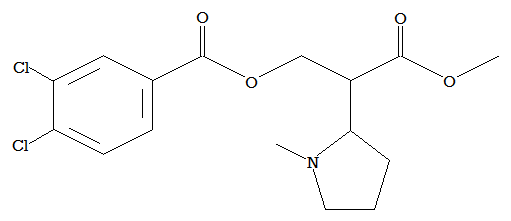
Take EPH here:
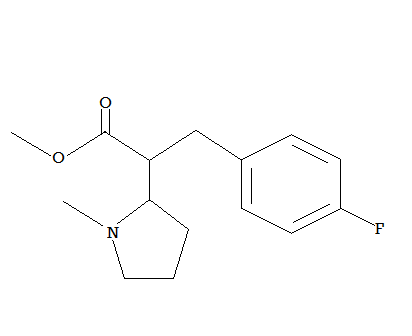
Now cut the bond between the C-alfa of the piperidinyl and the C-alfa of the Ph-CH-COOEt, merge the piperidinyl at the beta carbon with the C-alfa carbon of the PhCHCOOE to get a quaternary carbon..add a para substituted fluoro..you get this:
looks a little bit like arecoline skeloton
with extra Phenyl and saturated isnt it?
Now this compound is the single most potent stim that gets as close as it gets to cocaine..I mean almost carbon copy pharmacologically (same MAT inhibition ratios, distribution profile, logP, polar surface area..etc except it is at least 50X times more potent than cocaine (the 4-Bromo probably even more 100-200x?? way more potent and faster onset of action than MPH for sure.. Don't ask me how do I know..research it and you let me know!! (disclaimer: you can go to jail if you live in US or Canada because it is analog of schedule substance!! )
Oh! one more thing: it lasts longer than Ck or MPH (the COOEt beeing now attached to a quaternary carbon is more metabolically stable toward esterases hydrolysis .. way more stable than EPH or ck possibly T1/2> 8-10 hours at least 6 I bet!
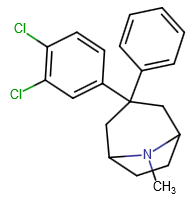
DotChem, could you draw this? Demerol-like isomer? Did you edit out the image? I think I've probably drawn it in theory before
EDITDotChem, could you draw this? Demerol-like isomer? Did you edit out the image? I think I've probably drawn it in theory before
I removed the image bc the country i live in just blanket ban any and all phenidates, their isomers, analogs and salts of isomers, analogs of isomers, isomers of analogs, salts of isomers of analogs, analogs of salt of isomers, isomers of salts of analogs of isomers..blablablablabla a couple of days ago..so technically it is illegal designing/suggesting anything that has ester, piperidine and phenyl in it that may or may not have stim activity..(I'll edit this post later as well.. DM me if you interested..
Here is really OLD paper on this
Last edited:
sekio
Bluelight Crew
- Joined
- Sep 14, 2009
- Messages
- 21,994
It's not illegal to draw or propose structures by the way. It's illegal to manufacture banned drugs, but banning the drug doesn't ban the literature discussion of it!so technically it is illegal designing/suggesting anything that has ester, piperidine and phenyl in it that may or may not have stim activity..(I'll edit this post later as well.. DM me if you interested..
Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
sekio
Bluelight Crew
- Joined
- Sep 14, 2009
- Messages
- 21,994
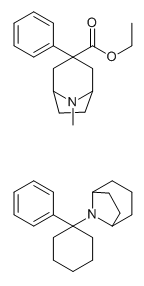
lol now that's look more and more like ligand for metal ions^
Edit: lowest energy conformation by MM-64 on above formentioned sekio's molucule with a metal ion
(I use a Cu2+ as representative for soft metal which likes N lone pair alot)
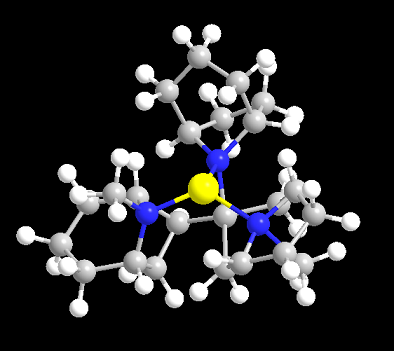
Edit: lowest energy conformation by MM-64 on above formentioned sekio's molucule with a metal ion
(I use a Cu2+ as representative for soft metal which likes N lone pair alot)

Last edited:
- Status
- Not open for further replies.