SeenSoFar
Bluelighter
- Joined
- Jun 21, 2013
- Messages
- 241
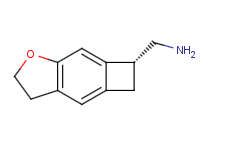
I would be very interested in seeing the first one explored in more detail. I'd also like to see the alpha-methylated analogue.
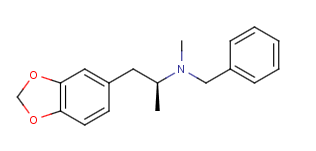
The second one I think would not have the effect you are aiming for. Alpha-methylated n-benzyl phenethylamine compounds are inactive from my understanding. The alpha-desmethyl might have some activity, but I believe it would probably be too bulky for the SERT. Still, it would be interesting to see what effect it did have...